I remember my first days as a new dentist and my introduction to the great debate over amalgam restorations vs. composite restorations. Today, we no longer place an amalgam or resin restoration without having a discussion with our patients over the advantages and disadvantages, strengths and weaknesses of amalgam vs. resin. This also applies to other materials, such as porcelain-fused-to-metal crowns vs. all ceramic crowns, vs. full metal crowns. Over the past 20 plus years, advances in medicine and dentistry have given rise to greater material options. 1 This holds particularly true in implant dentistry, where the use of biomaterials used to graft an extraction socket prior to implant placement, or used in conjunction to implant placement, have become an integral part of dentistry.
Biomaterials used in dentistry include bone grafts and barrier membranes. They come from various sources including that of human origin from the patients own body (autograft), from a human donor source (allograft), animal sources (xenograft), and synthetic sources (alloplast). In this great multicultural country of ours, we have patients in our office that come from all walks of life. These patients will present to our office with different ethical, religious, cultural, and philosophical beliefs, and may have a strong objection to a certain material. 1 Similarly, as dentists, we often have our own treatment and material preferences based on our own experience and biases. 1 We need to support our material and treatment selection with strong scientific evidence that supports our treatment recommendations. Evidence-based medicine can be defined as “the integration of the best research evidence with clinical expertise and patient values.” 2 We often face a dilemma and a tug-of-war when recommending a material, because the material that we feel provides the strongest outcome may be a material that a patient will object to, because of strong and very personal convictions. Patients always have the right to refuse a material, or refuse treatment despite our recommendations, even when supported by science.
We are all familiar with the process of informed consent. Informed consent involves more than just having a patient sign a consent form document. 3 Obtaining consent for treatment is a process. Unless an emergency, obtaining consent should take place before the day of treatment, and should involve communication between a patient and doctor. 13 According to Ontario’s Health Care Consent Act, 1996, discussions should include 3,4: Nature of treatment proposed; Expected benefits of treatment; Material risk and side effects of treatment, taking into account the individual circumstances of the patient; Alternatives, including other types of treatment including no treatment, and the likely consequences of declining the proposed treatment; Answers to any questions the patient has regarding the proposed treatment or alternatives.
The more complicated or risky our proposed treatment is, the more specific and detailed the consent process and documentation should be. 3 Sufficient time should be given to the patient to consider their options, such as treatment that is risky, elective, or esthetic in nature. 3
It is important to understand that obtaining consent for the biologic materials we select to use is equally as important as the consent we obtain for the procedural treatment we perform.
The following article will discuss the highlights of the current biomaterials available today and how it relates to the consent process.
Biomaterials In Implant Dentistry
Bone Grafting Material
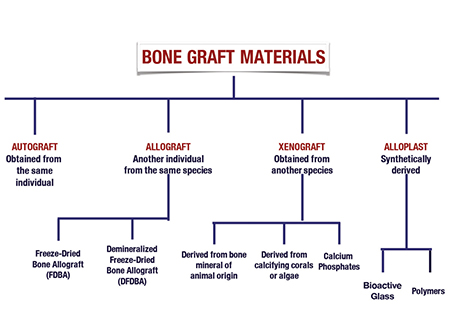
Bone graft materials used in dentistry today will fall under the category of (Fig. 1):
1. Autograft (derived from the patients own body).
2. Allograft (derived from a donor of the same species).
3. Xenograft (derived from a different species).
4. Alloplast (synthetic material).
Autografts
Autograft is bone derived and harvested from the same individual that is in need of the graft. It can be harvested from an intraoral source (ie. mandibular ramus, symphisis, tori) or an extraoral source (commonly the iliac crest). Considered the gold standard of bone grafting, this graft carries bone-stimulating growth factors, such as bone morphogenetic proteins, and viable osteogenic cells. 5 The disadvantage to the use of autografts is that it requires a secondary surgical site to harvest the bone, and often an inadequate amount of bone can be harvested. It has a high resorption rate, of up to 60% of the initial volume within six months. 6,7
There is growing interest in the use of Platelet Rich Plasma (PRP) and Platelet Rich Fibrin (PRF), used as an adjunct. PRP and PRF have been shown to help improve healing of an extraction socket, soft tissue healing, and defects around a dental implant. 8,9,10 Often performed chairside, the patient’s own blood is drawn through venipuncture, and the blood is then spun in a centrifuge at a specified revolution per minute (rpm). The result is a plasma (PRP) or fibrin matrix (PRF) containing a rich concentration of platelets and therefore increased growth factors, and increased angiogenesis. 8,9 A natural blood clot contains 94% red blood cells (RBCs), 5% platelets and 1% white blood cells (WBCs), while PRP and PRF contain 95% platelets. 8,10 PRF is a second-generation platelet concentrate, an improvement over traditionally prepared PRP. 8
Allografts
Allograft bone is derived from a donor of the same species. Often referred to as ‘donated human bone’, it is derived from a deceased donor. In implant dentistry, the most common forms are freeze-dried bone allograft (FDBA), and demineralized freeze-dried bone allograft (DFDBA). Allografts are available as cortical bone, cancellous bone, or a combination of both known as a cortico-cancellous mix. It is offered as a block or particulate.
The main advantage of allograft over autograft is the unlimited volume we can obtain, and it’s slower resorption rate.
Allografts must be obtained in the utmost ethical manner. The American Association of Tissue Banks (AATB) is an organization that provides accreditation to tissue banks. This membership and involvement of tissue banks is a voluntary one. 11 The advantage of membership is that it assists the tissue banks by providing guidelines for procurement, processing, quality control, and sterilization of bone grafts. 11 The AATB advocates the following exclusion criteria when selecting donor bone 12:
- donor from high risk-groups, as determined by medical testing.
- donor tests positive for HIV antibody by ELISA.
- autopsy of donor reveals occult disease.
- donor bone tests positive for bacterial contamination
- donor and bone tests positive for hepatitis B surface antigen, or hepatitis C virus.
- donor tests positive for syphilis.
In 2006, an unfortunate incident occurred in which the procurement of allograft was obtained by a bone bank without proper consent from the victims and their family members. 14 This lead to a massive recall in the bone grafting industry. It is important to understand the origin of the biomaterial we are using, how it was obtained, and the sterilization process. We hope a recall never occurs again. It is advised the tissue bank from which you obtained your bone source is accredited by the AATB.
Xenograft
Xenograft material is considered a bone substitute derived from another species. The most common xenograft bone substitute used in dentistry today is of bovine origin. However, bone substitutes of porcine origin are starting to find their way into the dental market today. Less commonly used xenograft material are derived from calcifying corals or algae. As with allograft, xenografts are processed so that all organic component is eliminated to remove the risk of transmission of disease.
Alloplast
Alloplast bone substitutes are completely synthetic. The materials most commonly available in dentistry today include calcium phosphates, bioactive glasses, and polymers. Calcium phosphates, mainly hydroxyapetite HA and beta-tricalcium phosphate (TCP), are most common because of their composition which resembles the inorganic phase of bone. 15 With increased and ongoing research on alloplast options, these materials may be an alternative option for patients who may be looking for a bone material other than a human or animal source.
Biologic Barrier Membranes
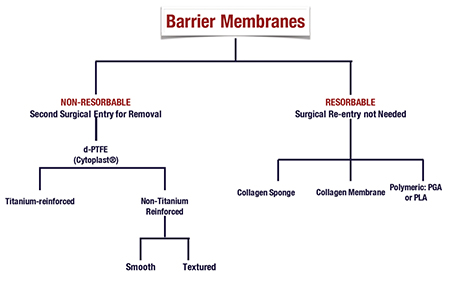
The use of barrier membranes for bone augmentation procedures prior to or simultaneously with implant placement, is considered the standard of care for such procedures. 5,16,17 With soft tissue growth occurring at a faster rate than bone, if allowed, soft tissue will populate a void area faster than bone. By adding a barrier membrane, we allow the exclusion of soft tissue and allow the population of osteblasts and precursors into the treatment area. Barrier membranes are utilized for the purpose of space maintenance, cell exclusion while still allowing blood cells to permeate through the membrane. The most common barrier membranes used today in dentistry can be categorized as resorbable barrier membranes and non-resorbable barrier membranes. Bioresorbable membranes have the advantage of resorption at a given time (depending on the design characteristics of that specific membrane) and therefore they do not require a second procedure for removal. Non-resorbable membranes, as the name implies, does not resorb and therefore require a second procedure for its removal (Fig. 3).
Fig. 1
Bioresorbable Membranes
Bioresorbable membranes used in dentistry today are derived from otherwise collagen of different animal sources, or synthetic aliphatic polyesters. 18
Synthetic aliphatic polyesters are made from otherwise polyglycolides (PGAs) or polylactides (PLAs). The advantage of these biomaterials is that they are completely biodegradable, reduced to carbon dioxide and water via the Krebs cycle. 18
Collagen barrier membrane used today are predominantly made from type I collagen (a major component of periodontal connective tissue), or from a combination of type I and type III collagen. 5 Sources include bovine tendon, bovine dermis, calf skin, or porcine dermis. 16 These membranes biodegrade through the enzymatic activites of macrophages and polymorphonuclear leukocytes. 5,19,20,21 The disadvantage to collagen membranes is their vast difference in biodegradability due to the crosslinking of the membranes. Therefore membranes are not created equally, and it is important to understand the resorption rate of your membrane before its use. A membrane that resorbs too quickly for your needs could result in inadequate bone formation.
Non-Resorbable Barrier Membranes
The first non-resorbable barrier membrane, expanded polytetrafluoroethylene (ePTFE), was developed in 1969, and marketed as Gore-Tex (Gore) by 1971. 5 PTFE is a synthetic fluoropolymer, getting its non-degradable properties from the bond between carbon and fluorine, incapable of enzymatic breakdown. 5 These membranes are also available with titanium reinforcement, providing greater structural integrity. This structural integrity allows for greater space maintenance resulting in greater formed bone beneath the membranes. 22 The disadvantage to ePTFE is the porosity of this membrane. This porosity makes this membrane prone to bacterial contamination, and soft tissue ingrowth. 23
Today, a newer generation of PTFE, known as high-density polytetrafluoroethylene (dPTFE) exists and now used in replacement of ePTFE. Composed of a micro-porous surface, it is impervious to bacteria, decreasing risk of infection when the membrane is exposed to the oral cavity. 23
In each package of bone graft material or membranes, identification stickers are included and used to identify the biomaterial (Fig. 2). Comparable to a fingerprint, it is a way to specifically identify the biomaterial that we use. These identification stickers are labeled with the reference and lot number of the material and can be placed in a patient’s chart. Most manufacturers offer a tissue tracking system, and often include a tracking form in the bone material packaging. Once the material is used, the office completes the tracking form with all the necessary information, and returns this form back to the manufacturer. Should a recall occur, they are able to identify your office immediately and notify you of a recall so that you may take the next necessary steps to inform your patient.
Fig. 2

Often times a patient will come to our office and refuse bone graft material of animal origin (xenograft). However, the refusal of xenograft extends beyond bone graft material. As you can see, most membranes are also created by the use of animal collagen. Having the knowledge of your material options will prevent this error from occurring. A different membrane will be needed for those that refuse a xenograft (Figs. 4, 5).
With so many increasing choices of biomaterials currently on the market, material selection can feel intimidating and quite daunting. Understanding the biologic principles of the materials, and your needs based on the complexity of the procedure will allow you to select the best material for your needs. Consider the safety of your product, affiliation with the AATB, evidence-based studies and research to evaluate the results (Figs. 4, 5).
Fig. 4

Fig. 5

Discussion
Patient’s opinions and perceptions of biomaterials used in medicine today were evaluated in a recent survey. 1 This survey indicated that allografts and xenografts elicited the highest refusal rate, while alloplasts and autogenous graft material had the highest acceptance. 1 The reasons behind the acceptance and objection of these materials were very unique to each individual in the survey. 1 This survey confirms the importance of an open dialogue about material choice selection with our treatment. Omitting this information means incomplete informed consent. Complicating matters further, the internet and social media has permeated all aspects of our life, from the way we communicate with each other, to the way we share information. I am sure we have all encountered patients who come to our office armed with information (whether accurate or inaccurate) about dental treatment, treatment options, and material options available today. Before moving forward with treatment, we often have to dredge through the information collected by the patient, and sort fact from fiction, allow our patient to digest the information we provide before moving forward.
In our practice, we often become very comfortable with long standing patients, and we will also encounter patients who wish a different level of involvement in the consent process. I cannot begin to count how many patients have said to me, “Doc, what would you do?” Although we will encounter some patients who want a quick and limited consent process, in no means does this absolve you from your duty.
A written consent does not prove complete consent but should be used to supplement your discussion. The consent form is often standard to your office but can be modified, with additions, highlights, underlining the key points relevant to your discussion, to support a consent process. Several patients will come into our office with English as their second language. The consent process may be difficult and often open to misinterpretation by the patient. Before any treatment is performed, I encourage these patients to come to each appointment with a family member that has an understanding of the English language, and can be used to interpret a conversation of treatment between doctor and patient. If your office is located in a strong cultural neighborhood where there is a predominant ethnic patient pool and language, a consent form drafted in the predominant language of your patients may be beneficial to assist in consent.
Understanding the biomaterials available today will allow a well-informed, and evidence-based discussion, taking into account individual patient beliefs and concerns. The trusting and long-standing relationship I have cultivated with my patients has developed over time, and is a direct result of the continued open communication and dialogue I have with every treatment recommendation. Patients appreciate it when they are included in the decision-making process, and when you have taken the time help them understand all aspects of treatment. This will also help alleviate any misunderstanding that can lead to future conflict. I feel this is a key cornerstone to the foundation of a lasting patient-dentist relationship and successful treatment. OH
Note: For a copy of Consent form for the use of bone graft and barrier membranes, please visit T.I.D.E. Institute website at www.tideinc.ca.
Oral Health welcomes this original article.
References
1. Fernandez R, Bucchi C, Navarro P, Beltran V, Borie E. Bone grafts utilized in dentistry: an analysis of patients’ preferences. BMC Medical Ethics 2015; 16: 71.
2. Sackett D, Straus S, Richardson W. Evidence-Based Medicine: How to Practice & Teach EBM. 2nd ed. London, England:Churchill Livingston;2000.
3. RCDSO, Practice Advisory. Informed Consent Issues Including Communication with Minors and with Other Patients Who May be Incapable of Providing Consent. Practice Advisory, August 2007.
4. Ontario Health Care Consent Act, 1996, S.O. 1996, c.2, Sched. A. Consent to Treatment.
5. Buser. 20 Years of Guided Bone Regeneration in Implant Dentistry, 2nd Edition. Quintessence Publishing 2009.
6. Widmark G, Andersson B, Inanoff CJ. Mandibular bone graft in the anterior maxilla for single-tooth implants. Int J Oral Maxillofax Surg 1997; 26: 106-109.
7. Widmark G, Andersson B, Inanoff CJ. Mandibular bone graft in the anterior maxilla for single-tooth implants. Int J Oral Maxillofax Surg 1997; 26: 106-109.
8. Saluja H, Dehane V, Mahindra U. Platelet-Rich fibrin: A second generation platelet concentrate and a new friend of oral and maxillofacial surgeons. Ann Maxillofax Surg. 2011 Jan-June 1(1): 53-57.
9. Bielecki T, Dohan Ehrenfest DM. Platelet-rich plasma (PRP and Platelet-Rich Fibrin (PRF): surgical adjuvants, preparations for in situ regenerative medicine and tools for tissue engineering. Curr Pharm Biotechnol. 2012 June; 12(7): 1121-30.
10. Sunitha R, Munirathnam N. Platelet-rich fibrin: Evolution of a second generation platelet concentrate. Indian J Dent Res. 2008;19:42–6.
11. Ham J, Miller PJ. Expanded polytetrafluoroethylene implants in rhinoplasty: Literature review, operative techniques, and outcome. Facial Plast Surg 2003; 19: 331-339.
12. “AATB: About Us”. American Association of Tissue Banks.
13. American Academy of Periodontology. Position Paper: Tissue Banking of Bone Allografts Used in Periodontal Regeneration. J Periodontol 2001; 72: 834-838.
14. Powell M, Segal D. In New York a Grisly Traffic in Body parts. The Washington Post. Saturday Jan 28, 2006.
15. Bohner M. Calcium orthophosphates in medicine: From ceramics to calcium phosphate cememts. Injury 2000; 31 (suppl 4): 37-47.
16. Bunyaratavej P, Wang HL. Collagen Membranes: A review. J Periodontol 2001; 72:215-229.
17. Hammerle CHF, Jung R. Bone augmentation by means of barrier membranes. Periodontology 2000 2003; 33: 36-53.
18. Hutmacher DW, Hurzeler MB, Schliephake H. A review of material properties of biodegradable and bioresorbable polymers and devices for GTR and GBR applications. Int J Oral Maxillofac Implants 1996; 11: 667-678.
19. Miller N, Pernaud J, Foliguet B, Membre H, Ambrosini P, Plombus M. Resorption rates of 2 commercially available bioresorbable membranes. A histmorphometric study in a rabbit model. J Clin Periodontol 1996; 23: 1051-1059.
20. Zhao S, Pinhold EM, Madsen JE, Donath K. Histological evaluation of different biodegradable and non-biodegradable membranes implanted subcutaneously in rats. J Craniomaxillofac Surg 2000; 28:116-122.
21. Owens KW, Yukna RA. Collagen membrane resorption in dogs: A comparative study. Implant Dent 2001; 10:49-56.
22. Jovanovic SA, Schenk RK, Orsini M, Kenney EB. Supracrestal bone formation around dental implants: An experimental dog study. Int J Oral Maxillofac Implants 1995; 10:23-31.
23. Cytoplast high-density PTFE membranes. Osteogenics.com
24. CDSPI, www.cdspi.com. Consent to Treatment Form.
About the Author
Dr. Tina Kokosis began her dentistry training at the University of Western Ontario, in London Ontario, where she received her Doctor of Dental Surgery Degree. She continued her education by moving to New York City, and obtained a Master of Science Degree in Periodontics at Columbia University. In addition to her private practice, Dr. Kokosis works at the University of Toronto Faculty of Dentistry as a part-time clinical instructor and is an instructor at the T.I.D.E institute in Toronto. Email: drtkokosis@torontoperio.com.
RELATED ARTICLE: Rethinking Health History When Bone Grafting












