(Updated March 2020)
The profession of dentistry in Canada, particularly in Ontario, has experienced some negative publicity in recent years as a result of practices that have been inspected and closed due to infection prevention and control breaches. In some of the cases, patients were notified that they may have been exposed to infectious diseases as a result of their dental visit. Although these breaches and patient exposures are rare, the news stories in Canada as well as reported breaches in the U.S., have created some concern on the part of patients as well as oral health care workers in Canada.
Several of these events involved improper documentation of sterilization of dental instruments and sterilizer monitoring. In response to these events, Public Health Ontario revised its Infection Prevention and Control (IPAC) checklist for dental practices, with the expectation that future breaches can be prevented. The checklist revision was completed in collaboration with the Royal College of Dental Surgeons of Ontario (RCDSO), the College of Dental Hygienists of Ontario (CDHSO), and the Ministry of Health and Long-Term Care (MOHLTC) and published in July 2019.1 This checklist, for reprocessing of dental/medical equipment/devices is based on the Public Health Ontario’s “Best Practices for Cleaning, Disinfection and Sterilization of Medical Equipment/Devices”(May 2013).2 In addition, the Royal College of Dental Surgeons of Ontario has updated their standards of care for infection control and prevention, which addresses instrument reprocessing and sterilizer monitoring and other elements of infection prevention and control (IPAC).3
IPAC Document
For dental practices who are not aware of the changes in regulations and guidelines, they may be unknowingly at risk of failure to comply with the regulations. Every dental practice should have copies of the following IPAC documents (Table 1).
Table 1

Dental/Medical Equipment/Devices July 2019
The following discussion will focus specifically on the requirements for recordkeeping and monitoring for instrument sterilization as part of reprocessing of dental instruments. It is critically important, however, that dental practices comply with all regulations and guidelines.
To effectively implement IPAC protocols in a dental practice, it is important to understand how the Public Health Ontario checklist applies, since there is a hierarchy, or degree of seriousness or risk in the checklist elements. This applies to both the Instrument Reprocessing checklist and the Core Elements checklist. These criteria are utilized during an inspection of a dental office but are also designed to be used as a self-audit or assessment tool to determine if there are any procedures that may need to be changed or implemented. Table 1 defines these compliance criteria:
Record Keeping
Documentation of the sterilization of dental instruments and devices is a critical element of a dental practice’s IPAC program. The following are the record keeping requirements, all of which fall into the high risk (HR) category:
o A log of test results during sterilization is maintained and reviewed. Information to be recorded includes:
- load control label (sterilizer number, load number, and date of sterilization)
- chart/printout of physical parameters of the sterilization cycle
- load contents
- person responsible for the sterilization cycle
- chemical indicator (CI) monitoring results – discussed later in this article
- biological monitoring (BI) monitoring results – discussed later in this article
Additional requirements that are medium risk (M) include:
o Efficacy testing and maintenance of equipment following the manufacturer’s instructions for use (MIFU)
- Efficacy testing of ultrasonic units and/or instrument washer/disinfectors
- Inspection, cleaning and routine maintenance of sterilizers
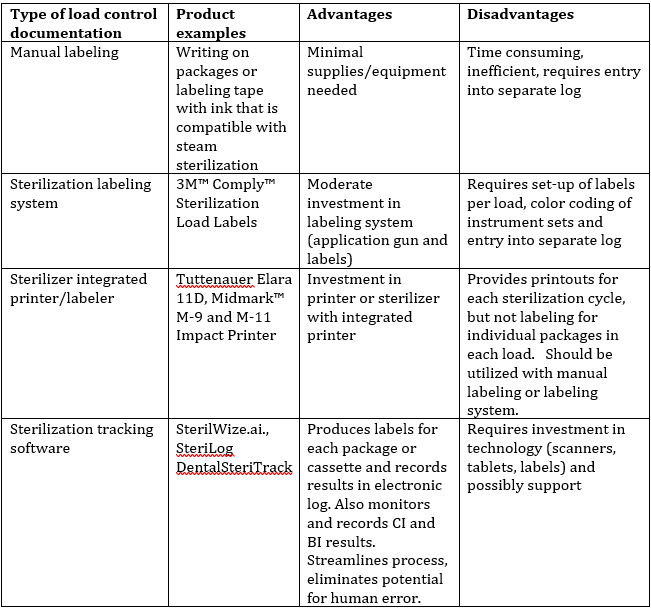
Load control information can be managed in several ways, beginning with manual labeling of packages and cassettes, utilizing a package labeling system that is designed to withstand the sterilization process, using sterilizers that have integrated printers that can produce labels with all the load control information, and utilizing sterilization tracking technology. The Table 2 lists the advantages and disadvantages of each type of system.
Table 2
If all the required information (listed above) is recorded, there are no specifications on how this is to be accomplished. It is, however, difficult and time consuming to use manual systems to record this information. In most dental practices, instrument reprocessing is performed by several members of the dental team, not necessarily dedicated to one team member, whose sole job is to manage instrument reprocessing. This can lead to lapses in protocol for recording of information, such as recording sterilization cycles in the log, or the types of instruments in the load. If the record keeping process is not efficient and effective, it is often overlooked or not complete. Manual systems require more time and attention to all the details needed with each pack or cassette of instruments, as well as each load of instruments processed. The results of both chemical indicators and biological indicators may be monitored, but not recorded. In this case, an inspector would assume that these procedures weren’t done, if there are no recorded results. Paper records can be misplaced or inadvertently destroyed by moisture and are unreliable at best.
If hand labeling of pouches or cassettes is the preferred method, care must be taken to follow the MIFU for the pouches or wraps for the appropriate place to write on the packages or wraps to prevent compromising them. It is also important to use pens that have been tested for used in sterilizers. Figure 1 shows the indicator to look for on a marking pen. This indicates that the pen has been validated for use according to the ASTM standard D4236,4 in the sterilization conditions and sterilization process used in the dental practice.5
Fig. 1

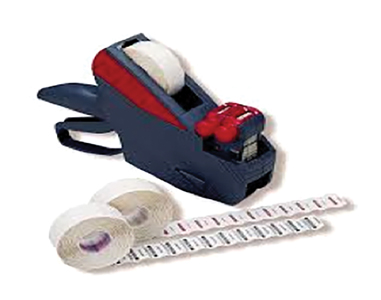
Utilizing a labeling system can streamline the process of placing labels on pouches or cassettes, but the user must make sure that the label can withstand the sterilization process. In addition, a system for color-code identification of the instruments in the load must be implemented (i.e. assigning a color label for each procedure set-up), but the information must still be entered into a log,
either on paper or electronically. Figure 2 is an example of a labeling system and colour-coded labels.
Fig. 2
Using a sterilizer with an integrated or attached printer that records information about the load, such as sterilizer number, sterilization parameters (time, temperature and pressure), the date and load number can also help to streamline the process, however, it does not identify the types of instruments in the load or record results of CI’s or BI’s. Figure 3 shows a sterilizer with an integrated printer.
Fig. 3

Sterilization Monitoring
Performing and recording sterilization monitoring procedures is a key element of the instrument sterilization process. Table 3 lists the sterilization monitoring and recording requirements for Ontario, as indicated on the checklist.
Table 3
A process challenge device is defined as a test device intended to provide a challenge to the sterilization process that is equal to or greater than the challenge posed by the most difficult item in the area of the package where steam would have most difficulty penetrating. PCD’s are available commercially or can be created in a dental office, using packaging, instruments BIs and CIs. Type 5 and Type 6 CIs measure all parameters necessary to achieve sterilization – time, temperature and pressure/steam penetration.
Considering all that is required for record keeping and monitoring of sterilizers, manual or combination of manual and automated systems appear to have great potential for failure of the system. As previously mentioned, it is labour intensive and time consuming to manually label and record all the necessary information, decreasing the potential for compliance as well as risk management.
Automating these processes by implementing sterilization tracking technology that integrates all the necessary elements for record keeping and monitoring results offers an excellent solution to enhancing compliance, reducing risks and increasing efficiency in instrument reprocessing. There are many of these types of systems available, but most are developed for use in hospital settings, however, there are several systems developed or adapted for use in dental offices. These systems are SterilWize – https://wize.ai/, SteriLog https://sterilog.ca/ and DentalSteriTrack https://steritrack.com, and Steritraces https://steritraces.com. While there are similarities in how the systems record information, there is a critical difference in labeling of the instrument pouches or cassettes. Steritraces and DentalSteriTrack are designed to have labels placed on the packages or cassettes after sterilization, which is not compliant with Public Health Ontario and Canadian Standards Group (CSA) document on Canadian medical device reprocessing.6 Labels must be placed on pouches or cassettes before they are placed in the sterilizer and remain securely attached during the sterilization process and when the instruments are stored. SteriLog and SterilWize, in contrast, are designed to allow labeling of the packages prior to sterilization to meet these requirements. These systems use bar-coded labels that can withstand the sterilization cycle and will remain on the packages in storage. If a system that requires labeling after sterilization is used there is a risk that in some cases the labels may not be placed before the instruments a put in storage, thus making the record keeping process incomplete.
Conclusion
There are many opportunities for errors in instrument reprocessing, any of which may cause a failure of the sterilization process and potentially put patients at risk of an infectious disease transmission. The RCDSO and PHAC have clear guidelines for instrument reprocessing, that if followed, will help to minimize the risk of processing errors. There are always the human factors to consider, however. OHCW’s may be inadequately trained and/or inexperienced, or they may be rushed due to scheduling or staffing issues. No matter what the cause, reducing the possibility of human error in instrument reprocessing can significantly reduce the risk of an infection prevention breach, as well as reduce liability to a dental practice or facility. Automating this process can reduce risk, increase compliance and enhance efficiency.
Oral Health welcomes this original article.
References
- Public Health Ontario, IPAC Checklist for Dental Practice Reprocessing of Dental/Medical Devices, July 2019, https://www.publichealthontario.ca/-/media/documents/checklist-ipac-dental-reprocessing.pdf?la=en (Accessed 12/10/19).
- Public Health Ontario – Provincial Infectious Disease Advisory Committee’s (PIDAC) “Best Practices for Cleaning, Disinfection and Sterilization of Medical Equipment/Devices”(May 2013) https://www.publichealthontario.ca/-/media/documents/bp-cleaning-disinfection-sterilization-hcs.pdf?la=en (Accessed 12/10/19).
- Royal College of Dental Surgeons of Ontario, Standard of Practice, “Infection Prevention and Control in the Dental Office (revised Nov. 2018), https://az184419.vo.msecnd.net/rcdso/pdf/standards-of-practice/RCDSO_Standard_of_Practice_IPAC.pdf. (Accessed 12/10/19).
- American Society for Testing Materials (recognized by CSA), available for purchase at: https://www.astm.org/Standards/D4236.htm (Accessed 12/10/19).
- CS Solutions, “Sharpies for Labeling; February 2013. https://cdn.hpnonline.com/inside/2013-02/1302-CSsolutions.html. (Accessed 12/10/19).
- Canadian Standards Association, Canadian Medical Device Reprocessing – Z-314-18. Standard available for purchase at https://www.scc.ca/en/standardsdb/standards/29301
(Accessed 12/10/19).
About the Author
 Mary Govoni is an internationally recognized speaker, author and coach on clinical efficiency, ergonomics, OSHA & HIPAA compliance, and team communication. She is a Certified Dental Assistant, Registered Dental Hygienist with over 45 years experience in the dental profession. Mary is a past president of the American Dental Assistants Association, a member of the American Dental Hygienists Association, a member of the Organization for Safety Asepsis and Prevention, the Academy of Dental Management Consultants and the Speaking Consultants Network.
Mary Govoni is an internationally recognized speaker, author and coach on clinical efficiency, ergonomics, OSHA & HIPAA compliance, and team communication. She is a Certified Dental Assistant, Registered Dental Hygienist with over 45 years experience in the dental profession. Mary is a past president of the American Dental Assistants Association, a member of the American Dental Hygienists Association, a member of the Organization for Safety Asepsis and Prevention, the Academy of Dental Management Consultants and the Speaking Consultants Network.