The implant restoration is an essential everyday treatment to replace missing teeth, advance function and enhance esthetics for patients in the general dental practice. An understanding of implant hardware design and placement for optimum clinical results is common knowledge for the dental practitioner. This is not the case when it comes to the understanding of bone grafting needs and procedures that are the foundations for implant treatment.
This article will address the basic principles for bone grafts in implant dentistry: the rationale, indications, locations, requirements, types, materials, and some guiding consensus on surgical techniques and materials. This will help clarify a somewhat murky new area for the general practitioner who needs to know more – whether it is to provide guidance to patients, or to increase understanding of the surgical protocols of implant treatment.
The Rationale for Bone Grafts
Placement of implants requires sufficient bone volume and biologic quality. This is due to the macro design of the implant, which demands certain dimensional properties for long-term success.1
Other factors which make bone grafting necessary are:
• The resorption of the edentulous ridge post extraction
• Presence of bony defects due to trauma or infection
• The need to place implants in strategic sites for functional and esthetic success. In esthetic areas, soft tissue requires a bony base since “soft tissue follows hard tissue”1
Treatment planning for bone graft placement requires the selection of an appropriate surgical technique and graft material. Poor planning or execution may lead to resorption of the graft material or its failure to integrate. In addition, the lost tissue may be replaced by fibrous tissue rather than functional bone.2
Grafts are suitable for a variety of clinical situations.
Locations/Indications for Bone Grafts in Implant Treatment
Bone graft materials are placed in different locations for various indications:1
• In alveolar sockets post extraction
• To refill a local bony defect due to trauma or infection
• To refill a peri-implant defect due to peri-implantitis
• For vertical augmentation of the mandible and maxilla
• For horizontal augmentation of the mandible and maxilla
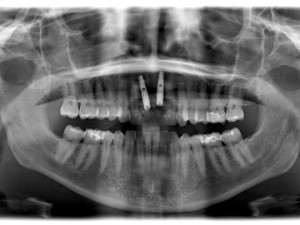
Following the extraction of a tooth, 40 to 60 percent of the original height and width of the surrounding alveolar bone is expected to be lost; the greatest loss is in the first two years3 (Fig. 1).
With this loss of hard and soft tissue, conditions are less favourable for the proper axial alignment of the implant for function and esthetics. To minimize alveolar atrophy post extraction, healing procedures termed “socket preservation” or “ridge preservation” have been developed. These procedures involve filling the socket with bone or bone substitute material, with or without a membrane. The objectives of ridge preservations are.4


• Filling the socket (wound care)
• Preservation of ridge volume (ridge preservation)
• New bone formation (osteogenesis)
Ridge preservation procedures seem to delay bone formation in the early healing phases5; however, studies show that procedures are effective with significantly lower ridge atrophy reported than in non-treated groups.6
There is always some bone loss after extraction, since the alveolar bundle bone into which the collagen fibres of the periodontium are anchored, is dependent on the presence of a tooth; this bone is always absorbed following tooth loss.7 The prime objective of ridge preservation is to diminish or completely eliminate the necessity of more invasive augmentation procedures in the future.
Techniques are available to effectively and predictably increase the width of the alveolar ridge (horizontal augmentation). Vertical augmentation techniques are not as predictable as those for horizontal augmentation and are subject to more complications.8
Bone grafts are more likely to succeed when the conditions at the recipient site are favourable and certain requirements are fulfilled.
Requirements for the Ideal Bone Graft
Bone healing and new bone formation after grafting occur through osterogenesis, osteoinduction and osteoconduction:3
• Osteogenic graft materials supply actual viable osteoblasts themselves
• Osteoinductive materials stimulate primitive mesenchymal cells brought in via the blood supply from adjacent bone or periosteum to differentiate into osteoblasts
• Osteoconductive materials merely act as a lattice or framework for cell growth, allowing osteoblasts from the wound margin to infiltrate the defect and to migrate across the graft. This brings a population of osteoblasts into the graft site
For the bone graft to be successful:2
1. Osteoblasts must be present at the site
2. Blood supply must be sufficient for nourishment
3. The graft must be stabilized during healing
4. The soft tissue must not be under tension
Bone is in a constant process of renewal with formation and resorption. During the first year of life almost 100 percent of the skeleton is replaced, while in adulthood the rate is closer to 10 percent per year.9 Remodeling enables bone to adapt functionally to changes in loading.
Osteoblasts – Only osteoblasts create new bone. For a graft to be successful, the graft matrix must contain or encourage population by osteoblasts. If there is an insufficient number of osteoblasts the graft will fail.2
Blood supply – Bone grafting is regeneration not repair. The term “repair” implies the regaining of lost tissue; regeneration is a biologic process where not only is the tissue regained, but also its form and function. This requires a good blood supply to the graft and surrounding tissue. Blood is needed for cell viability and clot formation. The clot serves as the initial matrix where cells migrate and then serves as anchorage for the osteoblasts.2
Graft stabilization – Mechanical stresses on the graft during healing can lead to disruption of the fibrin clot. Movement will cause fibrous tissue to fill the defect instead of bone. This is a form of repair and is not true regeneration. Fixation devices like GBR (guided bone regeneration) collagen membranes, titanium mesh and bone screws may be used.2
No tension on the soft tissue – Bone is the slowest growing tissue. Guided bone regeneration is based on the separation of the grafted site from the surrounding soft tissue. The GBR membrane keeps the faster growing tissues like epithelium, fibrous tissue or gingival connective tissue out of the defect allowing controlled regeneration to occur with vital bone formation.2 The application of bone graft material into the defect prevents the collapse of the collagen membrane and it acts as a place holder for new regenerating bone and an osteoconductive scaffold for the in growth of blood vessels and osteoblasts.10
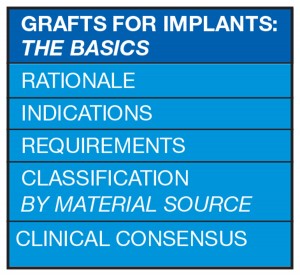
There are different types of bone grafts available, typically classified by the source of the material used.
Bone Graft Classification by Material Source
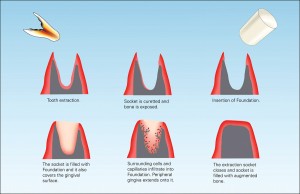
The autogenous graft (where tissue is transferred from one location to another in the same individual) is considered to be the gold standard. It is osteogenic, osteoinductive and osteoconductive.3 There is biological activity due to vital cells and growth factors. There is also no risk of disease transmission. However there is an increased risk of pain, infection, donor site morbidity, complexity in the surgical procedure, and a limited supply of bone3 (Fig. 2).
Bone substitute materials (BSM) were developed to counteract the difficulties of autogenous grafts. They can either replace autogenous bone entirely or expand the autogenous graft. Materials need to be effective for procedures both before insertion of the implants (time-delayed procedures) and for optimization of the recipient site at the time of implant placement (simultaneous procedures).1
Grafts are classified as:11
Autograft (autogenous graft):
Tissue transferred from one location to another within the same individual
Allograft:
A graft between genetically dissimilar members of the same species i.e. human tissue
Xenograft:
A graft taken from a donor of another species i.e. bovine, porcine etc
Alloplast:
Inorganic, synthetic or inert foreign material implanted into tissue
The autograft is the patient’s own bone. It is chiefly harvested intraorally or from the iliac crest. It is the ideal bone substitute since in contains living cells and human growth factors. It has greater osteogenic potential than any other bone substitute as well as inherent biocompatibility.12
The allograft can be derived from cadavers or living donors (tissue harvested from hip replacement surgery). It has natural bone composition and structure. This tissue is osteoinductive as well as osteoconductive but lacks osteogenic properties because of the absence of viable cells.12
A controversy exists as to the association of allogenic material and the risk of transmission of infections such as HIV, hepatitis B and C, prions, malignancies, systemic disorders or toxins.
Aggressive allograft processing gives it a less intense immunologic response, but reduces the osteoinductive properties. Frozen allografts induce stronger immune responses than freeze dried allografts, hence they are no longer used.12
The donor tissue is cleaned and then undergoes ultrasonics to remove blood and tissue components and to eliminate fat from the cancellous bone structure; this improves penetration of the surrounding tissues into the graft material.
Then chemical treatment denatures non-collagenic proteins, inactivates viruses and destroys bacteria. Further oxidative treatment denatures persisting soluble proteins and eliminates potential antigenicity. Dehydration preserves the structural integrity of the material. Final sterilization by gamma radiation ensures sterility.
Allografts are available in different shapes from demineralized bone matrix granules to complete bone segments. Granules can be used in socket preservation for future implant placement, ridge reconstruction for prosthetic therapy, filling osseous defects and maxillary sinus floor elevation.
Allograft bone segment blocks are a predictable and effective alternative to traditional autogenous block grafting and ridge augmentation.13 When very large areas need to be grafted, a shell of autogenous bone is often used as a biologic container; this creates the necessary space for the incorporation of the particulated bone graft material. The bone cells in the autogenous bone die within a few days and then the boneplate functions as a stable, avital, slowly resorbable membrane.14 Allogenic bone blocks can also be used for this shell technique as a substitute for autogenous bone. This avoids the time consuming harvesting and splitting of the autogenous bone blocks.
The space between the local bone and surrounding shell can be filled with a variety of different particulated bone grafting materials (autogenous, allogenic, xenogenic or alloplastic).
Histologic studies have shown no difference in the final stage of incorporation between allografts and autografts.15
The xenograft is derived from other organisms, mainly bovine. It provides long-term volume stability. Porous natural hydroxyapatite can be obtained from animal bones.
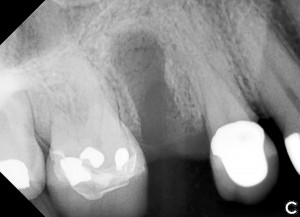
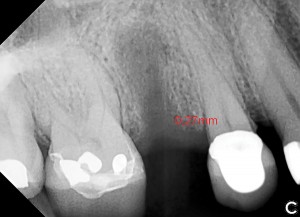
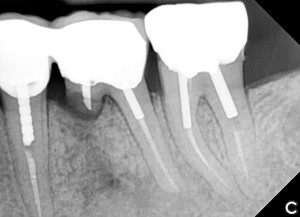
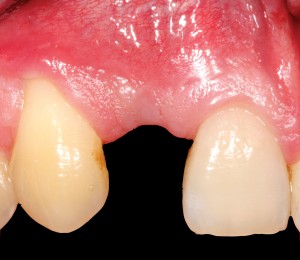
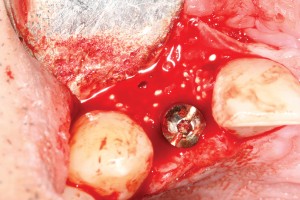
Bovine bone has a long well-documented tradition. It is deproteinized by heating to eliminate the risk of allergic reactions and disease transmission.16 The removal of all proteins transforms it into biologically derived hydroxyapatite ceramic. It is characterized by well-preserved 3D natural bone structure similar to human bone. The trabecular architecture with interconnecting pores allows for optimal in-growth of new vascularity.12 Guided osseous integration rather than rapid resorption leads to excellent volume stability of the graft with the formation of new bone on the highly structured bovine bone surface. The bovine bone xenograft is osteoconductive and is available in a range of volumes and particle sizes (Figs. 3 & 4).
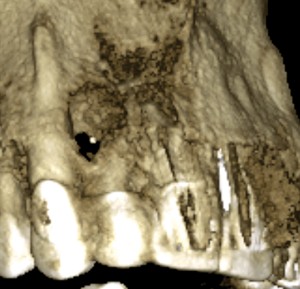
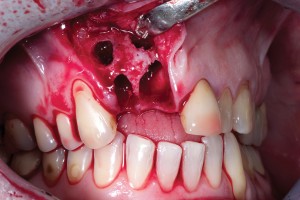
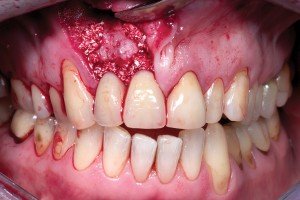


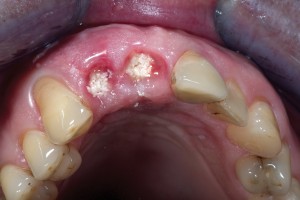
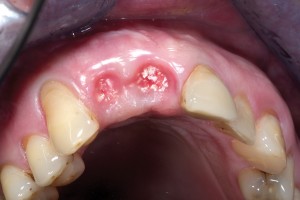
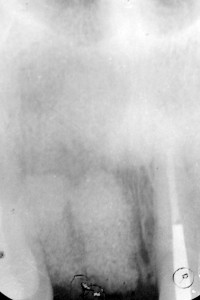
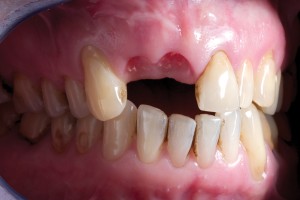

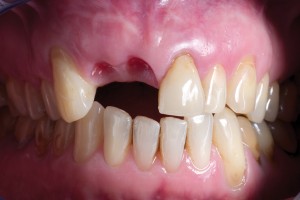
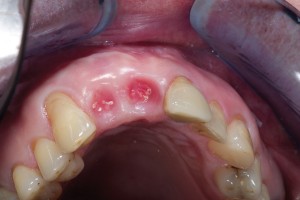



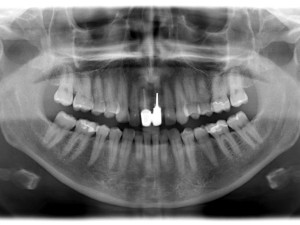
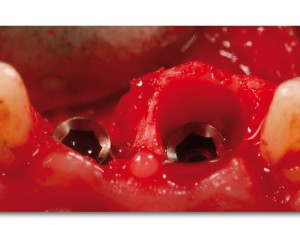
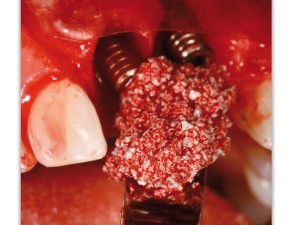



The above case is courtesy of Dr Henriette Lerner, Associate Professor University Iasi, HL-DENTCLINIC & ACADEMY, Baden-Baden, Germany and MIS Implants.
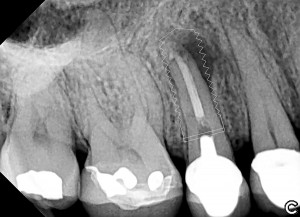
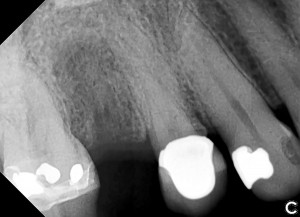
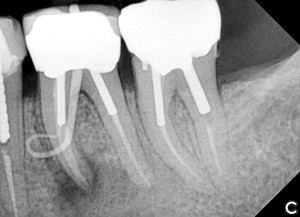
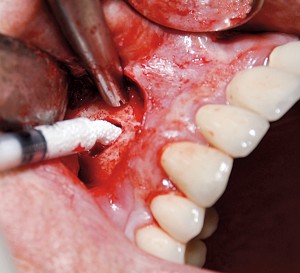
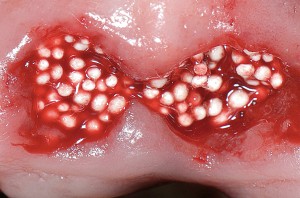
Another option is the use of bovine collagen. Untreated collagen (which acts as a scaffold) and heat-denatured collagen (which stimulates growth) are mixed, freeze-dried and cross-linked by heat. The material is then processed into a sponge block and formed into a bullet shape for easy placement into the extraction socket. Clinical studies show stimulation of new bone at an accelerated pace17 (Figs. 5,6 & 7).
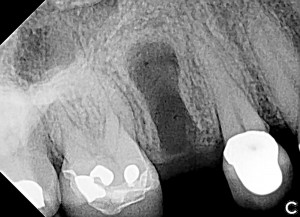

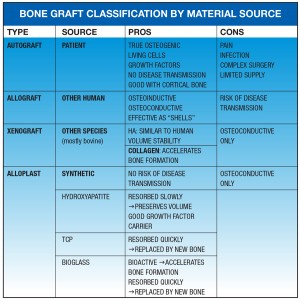
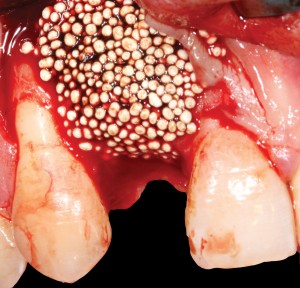
The alloplast is synthetically produced so there is no risk of disease transmission. The most common alloplastic materials are calcium phosphate based ceramics such as hydroxyapatite (HA) and tricalcium phosphate (TCP) (Figs. 8 & 9). Calcium phosphates are BIOACTIVE and RESORBABLE. They support attachment and proliferation of bone cells and undergo natural remodeling. There is an initial integration of the material into the surrounding bone matrix and then a gradual degradation. HA is incompletely resorbed while TCP is completely resorbed.
HA is the inorganic base bone substance that makes up two thirds of bone. HA ceramics are chemically nearly identical to natural HA.12
TCP is a calcium phosphate ceramic that is used as a synthetic scaffold substance in dentistry and orthopedics. Both TCP and HA have blood biocompatibility and osteoconductivity without immunogenic or toxic effects. However, they possess no osteogenic or osteoinductive properties and demonstrate minimal immediate structural support.12
HA and TCP differ in the biologic response created at the host site. TCP is removed from the implant site as bone grows into the scaffold; HA is more permanent.
HA’s slow solubility provides long term volume stability. It is also an excellent carrier of osteoinductive growth factors and osteogenic cell populations, adding to its value as a bioactive delivery vehicle.12
An ideal bone regeneration material should be resorbed in pace with new bone formation. The basic principle of using HA and TCP in combination is a balance between the stable HA which can be found years after implantation, and the fast resorbing TCP. The ratio between the two affects the resorptive properties of the graft material. A ratio between 65:35 and 55:45 of HA to TCP has been proven particularly suitable in many studies.18



The HA portion remains integrated in the newly formed bone, while the TCP part of the product is resorbed; it is replaced by new bone which imbeds itself within the remaining HA component creating a stable scaffold.4 Products with TCP alone are completely resorbed and replaced by bone within five to 15 months.4


Bioactive glass is another alloplastic bone substitute material. It is used extensively in orthopedics and dentistry. It is more reactive than inert materials like HA or TCP. Intrinsic properties of bioactive glass give it the ability to promote natural bone regeneration by releasing mineral ions.19 After reacting with blood it binds with bone and progressively releases silica ions. This stimulates osteoblast differentiation and proliferation.20, 21 Over time, it is fully absorbed and replaced by bone. When mixed with autogenous bone graft material, it doubles natural bone regeneration.22
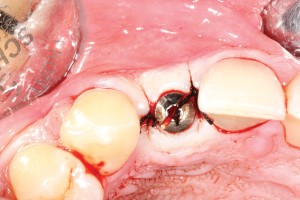
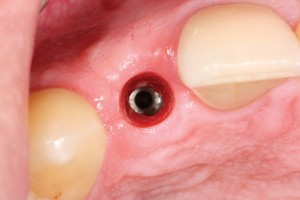
FIGURE 9J. Incisal view seven weeks after reopening the flap.
Different types of bone substitute materials can be combined and even hybridized to accommodate the needs of the clinical situation. Adjustment of material composition and physical characteristics allows for a wide range of resorption rates as well as physical forms such as powders, granules, pastes, blocks and even custom manufactured grafts. The selection of products allows the clinician to obtain optimal clinical outcomes.
Scientific foundations for bone grafting lead to practical clinical considerations. These are discussed below.
Consensus on Surgical Techniques and Materials
• The survival rates of implants placed into grafted areas are comparable with survival rates of implants placed into pristine bone.1
• Bone quality at the recipient site determines the type of graft material to be used. Cortical bone is inferior to cancellous bone at the recipient bed. Cells within cancellous bone are responsible for at least 60 percent of the patient’s bone healing capacity. The periosteum in a young, healthy patient contributes an additional 30 percent. Cells in the cortical bone are responsible for only 10 percent of bone healing. After extraction, when bone resorbs, cancellous bone shrinks relative to cortical bone. As the cancellous compartment decreases, the reservoir for osteoblasts does as well. Computerized tomography can reveal the ratio of cancellous bone to cortical at the recipient site prior to surgery. This ratio helps in the selection of the graft material as follows:
Only cortical bone–autograft
Cortico-cancellous–depends on predominant type
Mostly cancellous–everything is possible2
• Ridge preservation techniques are effective in limiting horizontal and vertical bone loss post extraction versus healing by blood clot alone.29
• Strong evidence shows that ridge preservation significantly maintains ridge width and height. Most graft materials are effective with only slight differences between them.29
• ‘External’ augmentation procedures, both horizontal or vertical, on the alveolar ridge are more difficult than ‘internal’ augmentation procedures in areas like the maxillary sinus.1
• Augmentation of vertical alveolar ridge defects exhibit higher complication rates than those for horizontal
defects.28
• For horizontal and vertical ridge augmentation procedures autogenous bone blocks result in higher gain than particulate materials. Survival rates of implants placed in horizontally and vertically augmented alveolar ridges are high.8
• Autogenous onlay bone grafting procedures are effective and predicable for the correction of severely resorbed edentulous ridges to allow implant placement. Survival rates are slightly lower than those of implants placed in native pristine bone.8
• There is a lack of long term information on the longevity of preserved ridges and the survival of the implants placed.29
And of course:
• Poor blood supply, trauma or extensive surgery in the area can worsen the prognosis.23
• Complications are higher in smokers.24, 25
• General diseases affecting bone metabolism like uncontrolled diabetes, radiation to head and neck, bisphosphonate therapy are at least relative contraindications for bone augmentation.26, 27
Conclusion
The subject of bone grafts for implant procedures is complex and confusing for the surgeon, let alone the restorative dentist and patient. This article has attempted to simplify and clarify the basics. Armed with this information, the general dentist can be a better judge of the techniques and materials used. This information can prepare the clinician for counseling patients on the surgical procedures to be performed at the specialist’s office or be the impetus to further exploration of simple bone grafting procedures that can be done in the
general practice.OH
Dr. Fay Goldstep has been a featured speaker in the ADA Seminar Series and has lectured at the American Dental Association, Yankee, American Academy of Cosmetic Dentistry, Academy of General Dentistry, and the Big Apple Dental conferences. She has lectured nationally and internationally on Proactive/Minimal Intervention Dentistry, Soft-Tissue Lasers, Electronic Caries Detection, Healing Dentistry and Innovations in Hygiene. She has served on the teaching faculties of the postgraduate programmes in Aesthetic Dentistry at SUNY Buffalo, University of Florida, University of Minnesota and University of Missouri-Kansas City. She is a Fellow of the American College of Dentists, International Academy for Dental-Facial Esthetics, American Society for Dental Aesthetics and the Academy of Dentistry International. Dentistry Today has listed her as one of the leaders in continuing education since 2002. She sits on the editorial board of Oral Health. Dr. Goldstep is a consultant to a number of dental companies and maintains a private practice in Markham, Ontario. She can be reached at goldstep@epdot.com.
Oral Health welcomes this original article.
REFERENCES
1. Klein MO, Al-Nawas B. For Which Clinical Indications In Dental Implantology Is The Use Of Bone Substitute Materials Scientifically Substantiated? Eur J Oral Implantol 2011; 4 (suppl): S11-S29
2. Smiler D, Soltan M. The Bone Grafting Decision Tress: A Systematic Methodology for Achieving New Bone. Implant Dentistry 2006; Volume 15, Number 2.
3. Wang H-M, Kiyonobu K, Neiva RF. Socket Augmentation: Rationale and Technique. Implant Dentistry 2004; Volume 13, Number 4.
4. Schug J, Kirste M, Huber A, Hollay HC, Troedhan A, Leventis MD. Post Extraction Alveolar Ridge Preservation: Scientific Background, Minimally Invasive Treatment Protocols and Expert Reports Using Alloplastic Biomaterials. Sunstar GUIDOR 2014;V 1.0.
5. Araujo M G, Liljenberg B and Lindhe J: beta-tricalcium phosphate in the early phase of socket healing: an experimental study in the dog Clin Oral Implants Res (2010) 21(4): 445-54.
6. Vignoletti F, Matesanz P, Rodrigo D, Figuero E, Martin C and Sanz M: Surgical protocols for ridge preservation after tooth extraction. A systematic review Clin Oral Implants Res (2012) 23 Suppl
5: 22-38.
7. Araujo M G, Liljenberg B and Lindhe J: Dimensional ridge alterations following tooth extraction. An experimental study in the dog J Clin Periodontol (2005) 32(2): 212-8.
8.Jensen SS, Terheyden H. Bone Augmentation Procedures in Localized Defects in the Alveolar Ridge: Clinical Results with Different Bone Grafts and Bone-Substitute Materials. The international Journal of Oral & Maxillofacial Implants; Volume 24, Supplement 2009.
9. Wheeless’ Textbook of Orthopedics. Data Trace Internet Publishing, LLC.
10. Rothamel et al. (2012). Clinical aspects of novel types of collagen membranes and matrices-Curent issues in soft and hard tissue augmentation. European Journal for Dental Implantologists.
11. Laney WR (ed). Glossary of Oral and Maxillofacial Implants. Berlin: Quintessence, 2007.
12. Kolk A, Handschel J, Drescher W, Rothamel D, Kloss F, Blessmann M, Heiland M, Wolff K-D, Smeets R. Current Trends and Future Perspectives of Bone Substitute Materials – From Space Holders to Innovative Biomaterials. Journal of Cranio-Maxillo-Facial Surgery 2012; 40: 706-718.
13. Gomes KU, Carlini JL, Biron C, Rapoport A, Dedivitis RA. Use of allogeneic bone graft in maxillary reconstruction for installation of dental implants. J Oral Maxillofac Surg. 2008 Nov;66(11):2335-8.
14. Etel F, et al. Theoretische Grundalagen der Knochentransplantation. In: Hieerholzer G, Zilch H; Transplantatlager und Implantatlager bei verschiedenen Operationen. Heidelberg: Springer, 1080:1-12
15. Urist MR, Bone: Formation by autoinduction. Science 150:893, 1965.
16. Murugan et al. (2003). Heat-deproteinated xenogeneic bone from slaughterhouse waste: Physico-chemical properties. Bull Mater Sci 26:523-528.
17. Rasubala L et al. (2010) Healing of Human Extraction Sockets After the Use of Foundation™, University of Rochester Medical Center. Eastman Institute of Oral Healt18h
18. Daculsi (1998). Biphasic calcium phosphate concept applied to artificial bone, implant coating and injectable bone substitute. Biomaterials 19:1473-1478.
19. Hoppe, A. et al. A review of the biological response to ionic dissolution products from bioactive glasses and glass-ceramics. Biomaterials. Elsevier, 2011, Vol 32. pp 2757-2774
20. Hench L.L. The story of Bioglass. J Matter Sci:Matter Med. Springer Science, 2006. Vol 17, P 967-978
21. Jones. J.R. Review of bioactive glass: from Hench to hybrids. Acta Biomaterialia. Elsevier. 2013 Vol. 9 pp. 4457-4486.
22. Oonishi, H et al. Quantitative comparison of bone growth behaviour in granules of Bioglass. A-W glass ceramic and hydroxyapaptite. J. Biomed Mater Res. John wiley & Sons, Inc., 2000. Vol 51
23. Landes CA. Implant-Borne prosthetic rehabilitation of bone-grafted cleft versus traumatic anterior maxillary defects. J Oral Maxillofac Surg 2006; 64: 297-307.
24. Wildmark G, Andersson B, Carlsson GE, Lindvall AM, Ivanoff CJ. Rehabilitation of patients with severely resorbed maxillae by means of implants with or without bone grafts: a 3- to 5-year follow-up clinical report. Int J Oral Maxillofac Implants 2001; 16:73-79.
25. Zitzmann NU, Scharer P, Marinello CP. Factors influencing the success of GBR. Smoking, timing of implant placement, implant location, bone quality and provisional restoration. J Clin Periodontol 1999;26:673-682.
26. GrÖtz KA. Zahnärztliche Betreuung von Patienten mit tumor-therapeutischer Kopf-Hals-Bestrahlung (Stellungnahme der DGZMK und DEGRO). Dtsch Zahnärztl Z 2002; 57:509-511.
27. Grötz KA, Kreusch T. Zahnärztliche Betreuung von Patienten unter/nach Bisphosphonat-Medikation, Stellungnahme der DGZMK, Stand 9/2006. DGZMK, 2006.
28. Rocchietta I, Fontana F, Simion M. Clinical outcomes of vertical bone augmentation to enable dental implant placement: a systematic review. J Clin Periodontol 2008;35(Suppl 8):203-215.
29. Darby I, Chen ST, Buser D. Ridge Preservation Techniques for Implant Therapy. The International Journal of Oral & Maxillofacial Implants; Volume 24, Supplement 2009.












