Introduction
The goal of modern dentistry is to restore the patient’s oral health to normal contour, function, aesthetics and speech. 1 The era of dental implants have ushered in a new treatment concept where the patients no longer have to use removable dentures, or sacrifice healthy adjacent teeth. The success and predictability of osseointegrated implants have forever changed the philosophy and practise of dentistry. 2
In the late 1950’s, Per Ingvar Branemark, a Swedish professor of anatomy studying blood circulation in bone and marrow, developed through a serendipitous finding a historical breakthrough in medicine: he predictably achieved an intimate bone to implant apposition that ordered sufficient strength to cope with load transfer. He called this phenomenon “osseointegration”. 3
Clinicians and patients have experienced much success with endosseous dental implants and in the world of dentistry is considered to have among the highest successful treatment outcomes. Successful events are always guided by a strict set of guidelines and protocols, when precisely planned and executed. A report published by the American Dental Council on Scientific Affairs recognized a consistently high rate of success in human clinical trials. Trials spanning from three to 16 years involving over 10,000 dental implants had an overall mean survival rate of 94.4% with a range between 76% to 98.7%. 4,5
Despite the long-term predictability of implants – biologic, technical and esthetic complications do occur in a certain percentage of cases. Based on numerous factors, the clinician will have a better understanding of the role of the device, procedural, anatomic, systemic, occlusal, microbial, immuno-inflammatory and genetic factors that may indicate or cause implant failures. With this understanding, clinicians may select appropriate cases or interventions that may enhance treatment outcomes for edentulous or partially edentulous patients. 6 Due to the remarkable success of dental implants, there is a growing interest in identifying factors associated with implant failure.
The scientific literature regarding the risk factors for implant failure is limited. As such, Esposito et al concluded that the treatment of biologic complications and failing implants lack systematic scientific validation and is based mainly on empirical experience and inference from in vitro findings on a trial and error basis. 7
Classification of Implant Failures
Implant failures can be classified as:
1. Early failure
2. Late failure
Early failures are said to occur before functional loading, whereas late failures occur following functional loading. The incidence of early failures have been reported in the range of 0.76% to 7.47% and late failures about 2.1% to 11.3%. 8 Most of the reasons cited for early implant loss were related to surgical trauma, infection, torque overload and certain local and systemic conditions. They can also be sub-classified as biological and mechanical failures.
Biological failures can be due to infections, where inadequate sterilization measures and poor aseptic surgical protocols were followed. Mechanical failures could be due to the breakage of the implant or implant screw due to excessive insertion torque values.
A biologic failure/early implant failure can be defined as the inadequacy of the host to establish or promote osseointegration.
One has to identify the signs of implant failure by thorough examination of the suspected site. One of the earliest and reversible signs of an affected implant will show inflammation of the surrounding soft tissue, a condition is termed “Peri-implant Mucositis”. This is a reversible clinical scenario that needs mechanical and chemical debridement to help resolve the inflammation surrounding the peri-implant mucosa. When there is bone loss accompanied by the inflammation of the peri-implant soft tissue, it is now termed “Peri-implantitis” as described by Albrektsson and Isidor. 9
Many of the features of peri-implantitis are similar to that of chronic adult periodontitis as described by Tonetti (1996) as “an inflammatory, bacterial infection-driven destruction of the implant supporting apparatus”. If not recognized early and treated with appropriate measures will lead to complete loss of osseointegration and thereby loss of the dental implant. 10 Therefore, an implant that is progressively losing bone anchorage, but is clinically stable can be defined as a “failing implant”. American literature describes another terminology “ailing implant” that is defined as a clinically stable implant affected with bone loss and pocketing.
From a therapeutic point of view, the distinction between failed implants, failing implants and biologic complications are critical. Clinically, lack of osseointegration is generally characterized by implant mobility. Therefore, in principle, a mobile implant is a “failed implant”. Implant failure is defined as the inability of an implant to fulfil functional, aesthetic and phonetic purposes (Phylant and Lekholm).
Implant failures can also largely be classified into four main categories:
1. Loss of integration
2. Positional failures
3. Soft tissue defects
4. Biomechanic failures
This article highlights and describes positional failures as one of the more common clinical reasons that could directly or indirectly relate to improper biomechanical force distribution for the loss of an implant.
Positional Failures
The most common type of failure is caused by poor treatment planning and/or poor surgical execution. Implant placement must be controlled and precise in order to support tooth like restorations. The final planned restoration should aide in the precise placement of the implant that will help with proper distribution of occlusal forces to the underlying basal bone. The incidence of this type error accounts for up to 10% of the failures. Avoidance is simply best by proper treatment planning, ideal implant site development, anatomic wax up based surgical guides and a good understanding of the restorative aspects of implant dentistry by the operating surgeon.
Malposition of the implant can lead to improper force distribution causing mechanical problems to the screw joint or in severe situations to the implant body. Screw loosening, breakage of implant components such as the prosthetic/abutments screws or the implant fixture are some of the common biomechanical errors involved with positional failures.
Case Report
A 68-year-old female patient had reported to the office with a complaint of a loose implant crown. The implant and crown was placed about three years ago and had a history of the implant crown debonding twice in the past two years. During the last 12 months, the patient has been experiencing constant food impaction and discomfort in the region. Her medical history noted that she was on Synthroid and Crestor with no other relevant medical conditions (ASA 2).
Clinical Exam and Diagnosis
Presence of a cement retained metal ceramic crown in #16 with food impaction was noted. (Figs. 1 & 2) The crown was mobile on palpation and depressible within the socket. Bleeding on probing with pocket depth over 10mm on all sides of the crown. Radiograph reveals radiolucency surrounding the entire body of the implant (Fig. 3). Presence of a 4-unit metal ceramic bridge on the opposing #45-#46-#47-#48 with good oral hygiene. Cervical abrasion was noted on the buccal of #15, with composite restorations on the cervical regions of #14 and #34.
Fig. 1

Fig. 2

Fig. 3

Treatment options presented included removal of the failed implant and:
1. No tooth replacement
2. Replace with single tooth partial denture
3. Replace with 3-unit conventional bridge
4. Socket graft and redo implant with screw retained restoration
After a detailed discussion on the risks, benefits, alternatives, costs and treatment time, the patient opted to proceed with re-doing the implant with a screw retained restoration.
Pre-Treatment Plan
Included pre-surgical oral hygiene, premedications (Amoxicillin 500 mg, 19 capsules, to take four capsules, one-hour prior to surgery and continue TID for five days, Ibuprofen 400 mg, 16 capsules, to take one capsule, one-hour prior to surgery and continue TID for five days, Dexamethasone 4 mg, seven tablets, to take two tablets, one-hourprior to surgery and continue BID for three days, Peridex mouth rinse, to rinse with 15 ml for 30 seconds, BID for two weeks post-surgery). The patient was well informed that no temporary tooth would be placed in site #16 until the final restoration eight months later.
Surgical Appointment
After pre-surgical preparation (patient is advised to brush her teeth, scrap her tongue thoroughly and rinse for 30 seconds with a cup of 0.12% chlorhexidine gluconate), local anaesthesia with 4% Articaine and 1:200,000 epinephrine was administered with a 33 gauge needle as a sub-periosteal infiltration, buccal and palatally with one carpule.
Implant crown #16 was removed with a conventional extraction forcep (Figs. 4 & 5) and a radiograph was taken (Fig. 6). Standard protocols for a phlebotomy (Fig. 7) was performed obtaining two vacuettes of venous blood (Fig. 8) to harvest platelet rich fibrin (PRF) derived growth factors. 2400rpm at 12 min spin protocol was performed (Fig. 9). The fibrin buffy coat layer (Fig. 10) was then separated from the tube (Fig. 11) and kept ready for placement along with the allograft.
Fig. 4

Fig. 5

Fig. 6

Fig. 7

Fig. 8

Fig. 9

Fig. 10

Fig. 11

A full thickness flap was elevated and the explanted site was observed to be filled with granulomatous tissue (Fig. 12). After thorough surgical debridement (Fig. 13) 0.25cc of cortico-cancellous chip allograft was mixed with finely cut PRF (Fig. 14) and placed into the socket (Fig. 15). The second PRF membrane was then placed over the surgical site and tucked under the buccal and palatal flaps (Fig. 16). A high-density PTFE membrane was trimmed and placed over the site (Fig. 16A) and gently tucked under the buccal and palatal flaps and sutured with 5-0 PGA resorbable sutures (Fig. 17). After completion of the procedure the patient kept placing her finger and tongue on the surgical site saying that she does not like the “feeling of the stitches” in her mouth and was very disturbed by it. For the patient’s comfort, a periodontal dressing (Fig. 18) was placed over the site to help prevent any disturbance from the patients finger or tongue. A post-surgical radiograph (Fig. 19) was taken prior to dismissal of the patient with post-surgical instructions.
Fig. 12
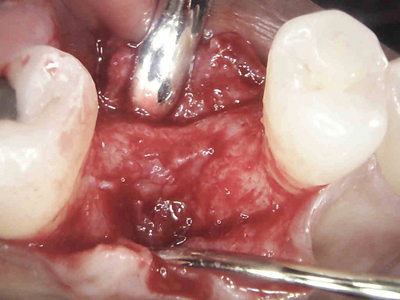
Fig. 13

Fig. 14

Fig. 15

Fig. 16

Fig. 16A

Fig. 17

Fig. 18

Fig. 19

The patient was reviewed after four weeks (Fig. 20) and the d-PTFE membrane was non-surgically removed with a cotton plier (Fig. 21). Home care instructions were re-inforced and patient was reviewed again four months later.
Fig. 20

Fig. 21

(mirror image).
Pre-implant clinical assessment (Figs. 22 & 23) was performed for the patient and radiographic assessment (Fig. 24) shows a homogenous trabecular pattern.
Fig. 22

Fig. 23

Fig. 24

On muco-periosteal flap reflection (Fig. 25) the surgical site shows a well-rounded preserved alveolar ridge, ideal for implant placement. Standard osteotomy drilling protocols were performed and a tapered implant 5.0 x 10mm (Fig. 26) was placed achieving primary stability and a conical wide healing abutment was placed and sutured. 12 weeks later the implant stability quotient value of 83 (Fig. 27) was confirmed with a radio frequency analyser, and a closed tray impression technique was recorded (Fig. 28). After 14 days, a screw retained non-precious metal ceramic restoration (Fig. 29) was inserted, verified intra-orally and radiographically. Occlusion was verified with an 8-micron articulating paper in MIP and excursive movement. Patient was happy with the outcome of the final restoration, functionally and aesthetically (Fig. 30). Interproximal cleaning aids were advised to maintain home care hygiene.
Fig. 25

Fig. 26

Fig. 27

Fig. 28

Fig. 29

Fig. 30

Discussion
Dental implants are currently the most ideal treatment option for the replacement of a single missing tooth. They have enjoyed a consistently high 95% success rate for over four decades and the patients and clinicians have benefited from this choice of treatment. However, when poor treatment planning, poor surgical execution and when a non-prosthetically driven implant restoration is provided, the long-term survivability of the implant restoration becomes questionable. The case report just discussed could have a number of possibilities associated to its failure. Malposition of an implant with increased cantilevering may cause excessive shear stress leading to implant failures. 11 The other possible cause (Fig. 5) presents with occlusal wear facets on the canine and first premolar that shows clear evidence of some form of parafunctional habits – evidence strongly suggest that such habits create an occlusal overload causing implant failures. 12 Different amounts of bone loss have been reported for different implant fixture designs. The shape of the body and surface condition of the implant body may affect the amount of strain distributed to an implant-bone interface. 13 As noted in Figures 3 and 4, the implant that failed was a single piece tapered narrow bodied implant that may not have been able to withstand bite forces in the first molar region especially given the poor position of the central core axis of the body of the implant (Fig. 5). Single piece implants have proven great success but needs more careful prosthetic planning and surgical execution. Given the anatomic region, the posterior maxilla (D4 bone) has minimal cortical and more cancellous bone-placement of a single piece implant will not allow for undisturbed healing during the phase of osseointegration. Bone is strongest under compressive forces, 30% weaker under tensile loads, and 65% weaker to shear forces (Fig. 31). 14 A better way to understand biomechanics is to review the literature introduced by Carl Misch–Stress Treatment Theorem–Sequence of Treatment Planning (Fig. 32). 15
Fig. 31

Fig. 32
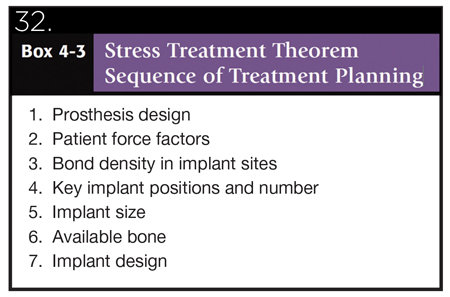
1. The final result (the prosthesis) should be visualized prior to the selection of the implant foundation.
2. Because stress equals force divided by the area to which the forces are applied, the amount of force is directly related to the amount of stress. There are several force factors to consider, including: (1) bruxism, (2) clenching, (3) tongue thrust, (4) crown height, (5) masticatory dynamics, and (6) the opposing arch. The forces applied to the restoration also differ by their (1) magnitude, (2) duration, (3) type, and (4) predisposing factors (e.g., cantilevers)
3. The density of bone is directly related to the strength of the bone. Misch et al. have reported on the biomechanical properties of four different densities of bone in the jaws. Dense cortical bone is 10 times stronger than the soft, fine trabecular bone. D2 bone is approximately 50% stronger than D3 bone.
4. The surface area of each implant is directly related to the width of the implant. Wider root form implants have a greater area of bone contact than narrow implants (of similar design), resulting from their increased circumferential bone contact areas. Each 0.25mm increase in implant diameter may increase the overall surface area approximately 5% to 10% in a cylinder implant body. Bone augmentation in width may be indicated to increase implant diameter by 1 mm when force factors are greater than ideal. In addition, it has been suggested that an increase in implant diameter may be more effective to reduce stress.
5. Implant macrodesign may affect surface area even more than an increase in width. A threaded implant with 10 threads for 10 mm has more surface area than one with five threads. A thread depth of 0.2 mm has less surface area than an implant with 0.4 mm.
6. Once the previous steps to the treatment plan sequence have been determined, the available bone in the potential implant sites is evaluated. If available bone is not present, bone augmentation or modification is required.
Conclusion
Clinical trials document a consistently high success rate for endosseous dental implants in partially and completely edentulous patients. Failures occur at a low rate but tend to cluster in those with common profiles or risk factors. These risk factors may be categorized as related to the implant device, surgical procedure, anatomy, systemic health, occlusion, microbial biofilm, host immuno-inflammatory response and genetics. Most of these factors appear to be more critical and needs to be addressed with top priority. Several risk factors can be minimized by a thorough understanding of all the factors responsible to a likelihood of an implant failure such as biomechanics and functional loading for an implant crown. With adequate experience and sound knowledge, the treatment plan can be designed to minimize their negative impact on the implant, bone, and final restoration. In identifying these factors and making appropriate interventions, clinicians can enhance dental implant success rates for better oral function, esthetics and patient well-being. OH
Oral Health welcomes this original article.
References
- Misch CE. Rationale for dental implants. Dental implant prosthetics. Mosby 2005, 1:1
- Klokkevold PR. Carranza`s Clinical Periodontology Part 8: Oral Implantology. 10th edition. Elsivier, Saunders 1071
- Branemark PI, Zarb GA, Albrektsson T. tissue integrated prosthesis, Chicago, Quintessence, 1985
- American Dental Association Council on Scientific Affairs. Dental Implants, an update. J Am Dent Assoc 2004; 135:92-7
- Behneke A, Behneke N, d`Hoedt B. Hard and soft tissue reactions to ITI screw implants. A 3 year year longitudinal results of a prospective study. Int J Oral Maxillofac Surg 1997;12:749-57
- Paquette D, Nadine B, Williams R. Risk factors for endosseous dental implant failure. Dent Clinics North Am 2006;5:361-374
- Esposito M, Hirsh J, Lekholm U, Thompsen P. Differential diagnosis and treatment strategies for biologic complications and failing oral implants: A review of literature. Int Oral Maxillofac Implants 1999;14:473-490
- Esposito M, Hirsh J, Lekholm U, Thompsen P. Biological factors contributing to failures of oseointegrated oral implants (II). Etiopathogenesis. Our J oral Sci 1998;106:721-764
- Quirynen M, d`Sote M, Steenberghe DV. Infectious risks for oral implants: A review of literature. Clin Oral Impl Res 2002;13:1-19
- Mombelli A, Van Oosten M, Schurch E, Lang NP. The microbiota associated with successful or failing osseointegrated titanium implants. Oral Microbiol Immunol 1987;2:145-151
- Lindquist JW, Rockler B, Carlsson GE: Bone resorption around fixtures in edentulous patients treated with mandibular fixed tissue integrated prostheses, J Prosthet Dent 59-63, 1988
- Naert I, Quirynen M, Van Steenberghe D et al: A study of 589 consecutive implants supporting complete fixed prostheses. Part II: prosthetic aspects, J Prosthet Dent 68:949-956, 1992.
- Karousis IK, Brägger U, Salvi G et al: Effect of implant design on survival and success rates of titanium oral implants: a 10 year prospective cohort study of the ITI Dental Implant System, Clin Oral Implant Res 15:8-17, 2004.
- Reilly DT, Burstein AH: The elastic and ultimate properties of compact bone tissue, J Biomech 8:393-405, 1975.
- Carl E.Misch. Stress Treatment Theorem for Implant Dentistry. Chapter 4, Contemporary Implant Dentistry, 3rd Edition, Mosby
About the Author
 Joshua Shieh is a third-generation clinician from a family heritage of over 80 years of dentistry. A Fellow of the International Congress of Oral Implantology, a Pre-Fellow of the Academy of General Dentistry, Member of the American Academy of Cosmetic Dentistry, Member of the Economics Committee at the Ontario Dental Association, Clinical Instructor at the Oral Rehabilitation Unit, Dept of Periodontics, Faculty of Dentistry at the University of Toronto, Clinical Instructor at The Institute of Dental Excellence, Program Director and Founder of the Academy of International Dental Education. He currently practices as a general dentist in Mississauga, Ontario with special interests focused on hard and soft tissue regeneration in implant dentistry. The author can be contacted by email: drjoshuashieh@gmail.com.
Joshua Shieh is a third-generation clinician from a family heritage of over 80 years of dentistry. A Fellow of the International Congress of Oral Implantology, a Pre-Fellow of the Academy of General Dentistry, Member of the American Academy of Cosmetic Dentistry, Member of the Economics Committee at the Ontario Dental Association, Clinical Instructor at the Oral Rehabilitation Unit, Dept of Periodontics, Faculty of Dentistry at the University of Toronto, Clinical Instructor at The Institute of Dental Excellence, Program Director and Founder of the Academy of International Dental Education. He currently practices as a general dentist in Mississauga, Ontario with special interests focused on hard and soft tissue regeneration in implant dentistry. The author can be contacted by email: drjoshuashieh@gmail.com.
RELATED ARTICLE: Risk Factors In Implant Dentistry: Unrestorable Implants
Follow the Oral Health Group on Facebook, Instagram, Twitter and LinkedIn for the latest updates on news, clinical articles, practice management and more!












