There is no doubt that the advent of dental implants has revolutionized the field of dental medicine. With the ability to esthetically and functionally replace single teeth or act as abutments for dentures, implants show not only versatility in application, but also innovation in the way in which we treat our patients. Implants also demonstrate improved patient approval as well, with more than 90% of patients stating complete satisfaction with implant therapy over the course of ten years.1 However, in order to place an implant into optimal functional and esthetic position, the quality and volume of the available alveolar bone must be taken into account. Failure to do so can have catastrophic results on ultimate implant outcome, as osseointegration and even patient acceptance depend on proper planning and placement of the implant. So, as clinicians, how do we approach the problem of a horizontally, vertically, or quality deficient alveolar ridge?
It has been well established in the literature that Guided Bone Regeneration (GBR) is the method of choice to augment bone in areas of alveolar ridge defects.2 Experimental studies in the 1980s by Nyman and Karring3,4 using barrier membranes paved the way for using the concept of cellular exclusion in periodontal tissue regeneration. They found that after the clot fills the space, barrier membranes allowed the osteogenic cells to colonize the region without competition from the overlying soft tissue cells. This technique was termed Guided Tissue Regeneration, which involved regeneration of bone, cementum and periodontal ligament around teeth.3-5 Soon after, many animal and human studies documented this concept for the regeneration of bone in the deficient alveolar ridge which was termed Guided Bone Regeneration.6-10
The purpose of guided bone regeneration (GBR) is to augment bone in a site that has lost bone due to alveolar ridge resorption, tooth extraction, or trauma. To achieve this, a wide range of barrier membranes and bone grafts have been documented in the literature with the most common approach being a combination of the two.
Membranes
A large variety of barrier membranes have been documented in the literature to be successful in guided bone regeneration. The main criteria required when selecting the appropriate membrane include the following characteristics; biocompatibility, tissue integration, cell occlussiveness, space making, and clinical manageability.11 The barrier membranes used for GBR procedures are classified as nonresorbable and resorbable. As for resorbable membranes, they can be further classified as natural or synthetic depending on the origin.
Expanded polytetrafluoroethylene (e-PTFE) membranes were the first barrier membranes documented for GBR. Classified as a nonresorbable membrane, it is a synthetic polymer with a porous structure that does not elicit an immune response by the tissues and is resistant to enzymatic and microbiological degradation.12 Additionally, the integration of titanium reinforcement within this type of membrane has given it increased mechanical stability and the ability to shape it according to the ridge. These characteristics showed great advantage in augmentation of challenging defects which have been documented in the literature.13,14 Furthermore, the introduction of dense-PTFE membranes, which are a less porous form of PTFE, has also shown great success in a large spectrum of bone augmentation procedures. The low porosity of d-PTFE prevents cell adhesion which makes membrane removal easier, while also making it less prone to bacterial incorporation.15,16 D-PTFE membranes have been documented in periodontal regeneration,17,18 ridge preservation procedures,19-21 and large GBR procedures.22,23
A wide range of resorbable membranes have been documented in the literature over the years. Examples include; collagen membranes (native and cross-linked), which are from natural origins, and synthetic membranes such as, polyglactin, polyurethane, polyglycolic acid and others. Resorbable membranes have been widely used in the literature for GBR procedures. The main advantages include: avoiding a second surgery for membrane removal; decreased patient morbidity; and more cost-effectiveness. On the other hand, some disadvantages discussed include; reduced barrier function over time; membrane resorption process may interfere with healing; and lack of membrane stability and rigidness which therefore, requires additional supporting materials to prevent membrane collapse.2
It is important to note that the use of resorbable membranes often requires primary closure of the wound in order to prevent premature loss of the membrane. In fact, it was shown that higher postoperative discomfort and a more coronally displaced mucogingival junction resulted from this additional tissue release for primary closure.24 On the other hand, many studies have documented successful ridge augmentation achieved by the use of d-PTFE membranes in simple or complex extraction cases without primary closure.20,21,25
Bone Grafts
Autogenous bone has been considered the gold standard for bone augmentation procedures, however, due to the need for a donor site, limited graft availability, and unpredictable graft resorption rate, other bone graft substitutes have gained popularity by most clinicians. Bone grafts are divided based on the origin into four groups: autografts (from the same individual), allografts (another individual of the same species), xenografts (from another species), and alloplasts (synthetically produced). Bone grafts are expected to fulfill certain criteria such as: biocompatibility; osteoconductivity; mechanical stability; biodegradability; and replacement with the subject’s own bone over time.2
With regards to xenografts; deproteinized bovine-derived bone mineral has been well-documented in the literature for GBR procedures.2,26-29 In fact, a recent systematic review reported less graft resorption with the use of xenografts alone or added to autogenous block or particulate graft, compared to using autogenous graft alone.30
On the other hand, allografts have been well-documented in post-extraction ridge preservation and GBR procedures.20,21,25, 31-33 Histologically, demineralized forms of allografts have been shown to result in higher percentages of vital bone formation and fewer residual graft particles compared to mineralized allografts, however, clinically no significant difference in ridge dimensions have been reported.34
The majority of systematic reviews on bone augmentation procedures have not concluded which barrier membrane and bone graft are superior to the others. This is partly due to the large variation in techniques and materials used in different studies which makes comparisons difficult. However, the literature has clearly documented the feasibility and predictability of guided bone regeneration whether in post-extraction scenarios or severely compromised ridge defects. It is the clinician who determines the appropriate technique and materials based on the clinical situation at hand. The following case report demonstrates a very common occurrence in daily practice and how it was managed by the clinician in a safe and predictable manner.
Case Report: Clinical Presentation
A 44-year-old Hispanic female non-smoker with non-contributory medical history presented to the periodontist office with a chief complaint of “I was recommended a dental implant to replace this broken tooth”. Initial clinical examination revealed tooth #36 presented as remaining roots with generally healthy surrounding gingival tissues and teeth (Fig. 1). The patient had good oral hygiene and opposing tooth #26 presented with slight supraeruption, however, adequate interocclusal space was present. Periapical radiograph of #36 revealed relatively short mesial and distal remaining roots with evidence of previous endodontic treatment. Bitewing radiograph of the region revealed mild bone loss involving the adjacent teeth (Fig. 2). These initial clinical and radiographic findings sway the clinician toward immediate implant placement, however, it is critical to further examine the case with a limited view CBCT prior to discussing the treatment plan with the patient.
Fig. 1A
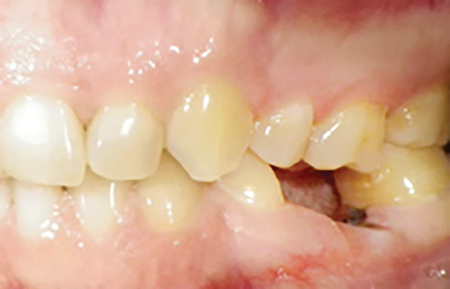
Fig. 1B

Fig. 2A
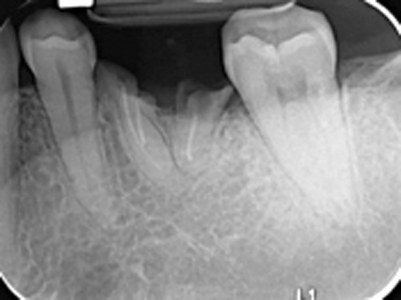
Fig. 2B

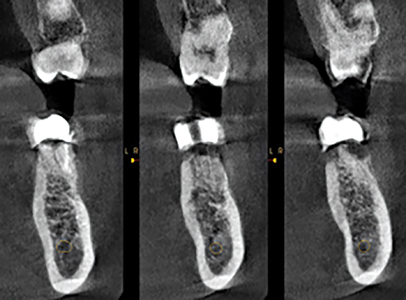
A limited view CBCT was obtained and revealed detailed information regarding tooth #36 and the surrounding anatomy as seen in Figure 3. The implant radiographic guide fabricated by the referring dentist can be seen in the CBCT scan which provided information about the proposed final implant crown position. There was ample bone available apical to the remaining roots up to the inferior alveolar nerve which is favorable for immediate implant placement, however, as seen on the axial sections of the CBCT, while a thick lingual plate was present, there was no bone on the entire buccal aspect of the remaining roots. Knowing this information prior to the surgery allowed for proper discussion and planning of the case with the patient. The patient was informed of the likelihood of postponing the implant and needing a bone augmentation procedure in the event that a compromised socket was present after removal of the tooth. Prior to the surgery, informed consent was provided by the patient for both possible procedures, immediate implant placement or bone augmentation with delayed implant placement.
Fig. 3A
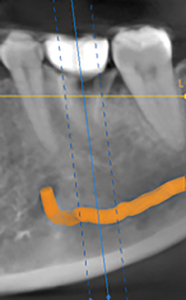
Fig. 3B
Case Management
After administration of anesthesia via an inferior alveolar nerve block and long buccal injection, periotomes were used around remaining roots of #36 and the root tips were elevated and then extracted with root forceps. Gentle curettage of the socket and irrigation with saline was performed. Inspection of the socket walls revealed absence of the buccal plate, therefore, reflection of the gingival tissues for better visualization of the socket was performed. A 15c blade was used for intrasulcular incisions on the buccal aspect, followed by full thickness elevation of the buccal gingiva was performed until the entire defect was exposed. The socket had a thick intact lingual plate, however, a large part of the buccal plate was missing as seen in Figure 4. Therefore, the patient was informed of the decision to perform bone augmentation at this stage and postpone the implant placement after the site has healed. For educational purposes of this article, a virtual implant was placed on the intraoperative photo as seen in Figure 4E to appreciate how much implant surface would be exposed if an immediate implant was placed at this time.
Fig. 4A

Fig. 4B
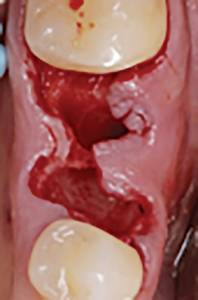
Fig. 4C

Fig. 4D
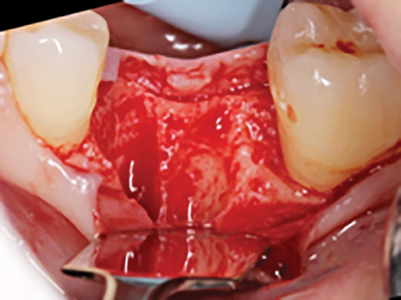
Fig. 4E

Initially, additional curettage of the socket was performed and bleeding points were initiated with a small round bur in the walls of the socket. Then a non-resorbable dense PTFE membrane (Osteogenics Cytoplast Membrane) was placed on the buccal aspect extending apically 2-3mm beyond the defect. The socket was then grafted with a particulate allograft (Zimmerbiomet Puros Cortical Particulate Allograft – Particle size 0.25-1.0mm) and covered with the membrane that also extended slightly under the lingual flap which was tunneled to allow for this maneuver. The membrane was stabilized initially with sutures in a cross-mattress style, followed by three single interrupted sutures placed to approximate the gingival flaps without attempting primary closure as seen in Figure 5. All sutures used were non-resorbable PTFE sutures.
Fig. 5A

Fig. 5B
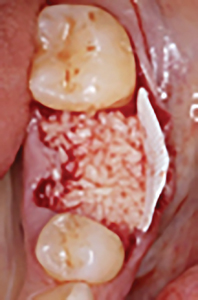
Fig. 5C

The patient was instructed to follow a cold liquid diet for the first 24 hours, followed by a soft diet for the remaining week and eat on the right side only. Patient was instructed to refrain from oral hygiene practices in the surgical site while rinsing with 0.12% chlorhexidine gluconate (three times daily) for 2 weeks, take 500 mg amoxicillin (every 8 hours) for 7 days and 400 mg ibuprofen or 500mg acetaminophen (every 6-8 hours) as needed for discomfort. At the 1-week postoperative visit, there was slight plaque accumulation and the gingival tissues were mildly erythematous and edematous, however, there was no sign of infection or complications. One suture which was nonfunctional was removed and the membrane was de-plaqued using a Q-tip soaked in 0.12% chlorhexidine as seen in Figure 6.
Fig. 6A
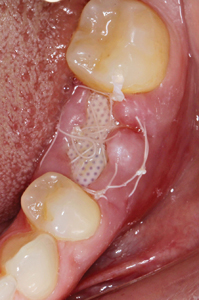
Fig. 6B

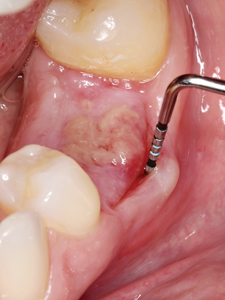
The patient returned at the two-week postoperative visit for suture removal and the membrane was left in place based on recommendation from the literature that removal is after four weeks.20 However, the patient did not return for membrane removal until seven-weeks postoperatively. The membrane was heavily covered in plaque, and the surrounding tissues were moderately erythematous and edematous. The membrane was tightly bound deep underneath the buccal tissues, however, removal was not difficult and the patient experienced no discomfort or bleeding during the removal process. The occlusal aspect of the socket was covered in keratinized gingiva and a gingival invagination in the buccal aspect was evident which reached 9 mm in depth at the mid-buccal region as seen in Figure 7. Inspection and palpation of the buccal aspect revealed no suppuration or signs of infection. The patient was instructed to continue rinsing with 0.12% chlorhexidine and avoid contact with this region for an additional week.
Fig. 7A

Fig. 7B
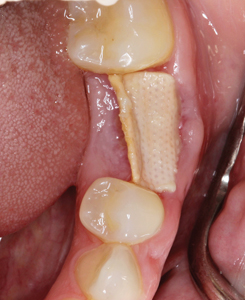
Fig. 7C
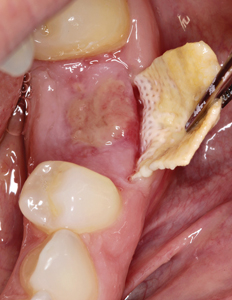
Fig. 7D
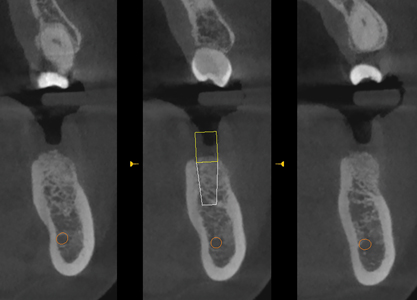
The patient returned for CBCT evaluation three months postoperatively as seen in Figure 8. A virtual implant was placed in order to determine if available bone is present for implant placement. The virtual implant shown in the figure is 5.0 mm in diameter and 10.0 mm in length with a tapered design. Adequate bone was present in all dimensions surrounding the virtual implant and on the axial sections we can see the grafted bone played a large role in augmenting the height and width of the ridge.
Fig. 8A

Fig. 8B
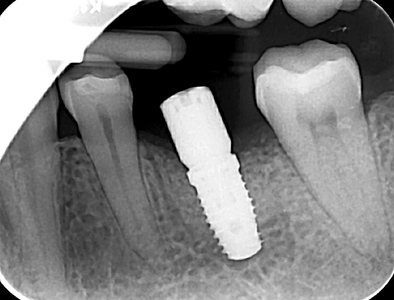
Implant placement was instigated 4 months post-extraction and bone augmentation. After administration of anesthesia via an IANB and long buccal injection, full thickness flaps were reflected and complete healing of the ridge was evident with no loose graft particles detected. The osteotomy was prepared and a 5.0mm diameter by 10.0mm length implant (Biomet Osseotite Tapered Certain Prevail) was placed with primary stability at 45Ncm, a healing abutment (Biomet BellaTek Encode Two-Piece Healing Abutment) was connected, and no additional grafting was necessary. Resorbable chromic gut sutures were used to approximate the flaps as seen in Figure 9. A periapical radiograph was taken immediately after the procedure as seen in Figure 10.
Fig. 9A
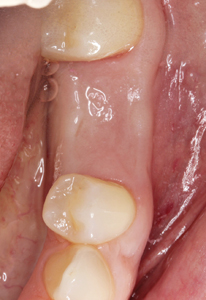
Fig. 9B
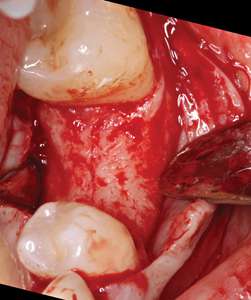
Fig. 9C

Fig. 10
The patient was instructed to follow a cold liquid diet for the first 24 hours, followed by a soft diet for the remaining week and eat on the right side only. The patient was instructed to refrain from oral hygiene practices in the surgical site while rinsing with 0.12% chlorhexidine gluconate (three times daily) for two weeks, take 500 mg amoxicillin (every eight hours) for seven days and 400 mg ibuprofen or 500 mg acetaminophen (every eight hours) as needed for discomfort.
Clinical Outcomes
Healing was uneventful with no complications. Figure 11 represents the healing at the two-month postoperative visit. On the occlusal photo, we can appreciate the healthy buccal contour maintained and the adequate keratinized gingival tissue surrounding the implant. A radiograph was taken at the three-month postoperative visit and at that time and the patient was sent back to the restorative department for restoration of the implant (Fig. 12).
Fig. 11

Fig. 12

Due to the patient’s busy work schedule, she didn’t return to the referring dentist until eight months later for impressions and delivery of the implant provisional. Figure 13 shows the final the radiographs taken by the referring dentist at the time of delivery of the screw-retained implant crown which was approximately 10 months post-implant placement.
Fig. 13A

Fig. 13B

The patient returned to the prosthodontist for a follow-up of implant #36 after six months in function. The patient was satisfied with the treatment rendered and had no concerns. Clinical inspection revealed healthy tissues surrounding the implant and radiographic examination showed stable bone levels with no sign of pathology as seen in Figure 14.
Fig. 14A
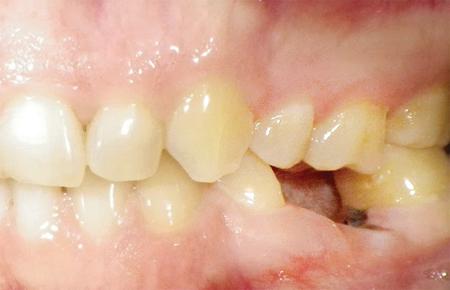
Fig. 14B
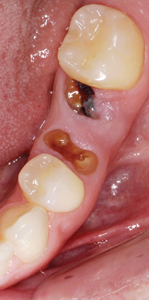
Fig. 14C
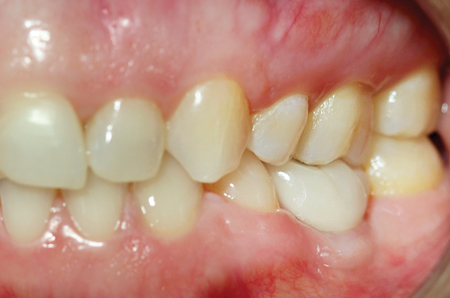
Fig. 14D
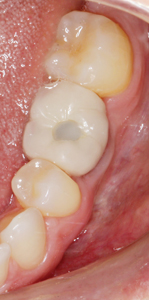
Fig. 14E
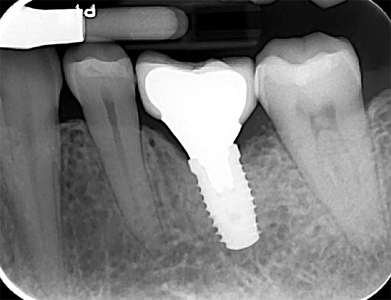
Fig. 14F

Oral Health welcomes this original article.
References
- Yao J, Tang H, Gao XL, McGrath C, Mattheos N. Patients’ expectations from dental implants: a systematic review of the literature. Health and quality of life outcomes. 2014 Dec;12(1):153.
- Benic GI, Hämmerle CH. Horizontal bone augmentation by means of guided bone regeneration. Periodontology 2000. 2014 Oct;66(1):13-40
- Karring T, Nyman S, Lindhe J. Healing following implantation of periodontitis affected roots into bone tissue. J Clin Periodontol 1980: 7: 96–105.
- Nyman S, Karring T, Lindhe J, Planten S. Healing following implantation of periodontitis-affected roots into gingival connective tissue. J Clin Periodontol 1980: 7: 394–401
- Nyman S, Lindhe J, Karring T. Reattachment – new attachment. In: Lindhe J, editor. Textbook of clinical periodontology. Copenhagen: Munksgaard, 1989: 450–473
- Dahlin C, Linde A, Gottlow J, Nyman S. Healing of bone defects by guided tissue regeneration. Plast Reconstr Surg 1988: 81: 672–676
- Dahlin C, Sennerby L, Lekholm U, Linde A, Nyman S. Generation of new bone around titanium implants using a membrane technique: an experimental study in rabbits. Int J Oral Maxillofac Implants 1989: 4: 19–25
- Buser D, Bragger U, Lang NP, Nyman S. Regeneration and enlargement of jaw bone using guided tissue regeneration. Clin Oral Implants Res 1990: 1: 22–32
- Lang NP, Hammerle CH, Bragger U, Lehmann B, Nyman SR. Guided tissue regeneration in jawbone defects prior to implant placement. Clin Oral Implants Res 1994: 5: 92–97.
- Wachtel HC, Langford A, Bernimoulin JP, Reichart P. Guided bone regeneration next to osseointegrated implants in humans. Int J Oral Maxillofac Implants 1991: 6: 127–135
- Hardwick R, Scantlebury T, Sanchez R, Whitley N, Ambruster J. Membrane design criteria for guided bone regeneration of the alveolar ridge. In: Buser D, Dahlin C, Schenk R, editors. Guided bone regeneration in implant dentistry. Chicago, IL: Quintessence, 1994: 101–136.
- Becmeur F, Geiss S, Laustriat S, Bientz J, Marcellin L, Sauvage P. History of teflon. European urology. 1990;17:299-300.
- Chiapasco M, Abati S, Romeo E, Vogel G. Clinical outcome of autogenous bone blocks or guided bone regeneration with e-PTFE membranes for the reconstruction of narrow edentulous ridges. Clin Oral Implants Res 1999: 10: 278– 288.
- Simion M, Jovanovic SA, Trisi P, Scarano A, Piattelli A. Vertical ridge augmentation around dental implants using a membrane technique and autogenous bone or allografts in humans. Int J Periodontics Restorative Dent 1998: 18: 8–23.
- Bartee, B.K. (1998) Evaluation of a new polytetrafluoroethylene guided tissue regeneration membrane in healing extraction sites. Compendium of Continuing Education in Dentistry 19: 1256–1264
- Barber, H.D., Lignelli, J., Smith, B.M. & Bartee, B.K. (2007) Using a dense PTFE membrane without primary closure to achieve bone and tissue regeneration. Journal of Oral and Maxillofacial Surgery 65: 748–752
- Lamb, J.W., 3rd, Greenwell, H., Drisko, C., Hender-son, R.D., Scheetz, J.P. & Rebitski, G. (2001) A comparison of porous and non-porous teflon membranes plus demineralized freeze-dried bone allograft in the treatment of class II buccal/lingual furcation defects: a clinical reentry study. Journal of Periodontology 72: 1580–1587.
- Walters, S.P., Greenwell, H., Hill, M., Drisko, C., Pickman, K. & Scheetz, J.P. (2003) Comparison of porous and non-porous teflon membranes plus a xenograft in the treatment of vertical osseous defects: a clinical reentry study. Journal of Periodontology 74: 1161–1168.
- Hoffmann, O., Bartee, B.K., Beaumont, C., Kasaj, A., Deli, G. & Zafiropoulos, G.G. (2008) Alveolar bone preservation in extraction sockets using non-resorbable dPTFE membranes: a retrospective non-randomized study. Journal of Periodontology 79: 1355–1369
- Fotek, P.D., Neiva, R.F. & Wang, H.L. (2009) Comparison of dermal matrix and polytetrafluoroethylene membrane for socket bone augmentation: a clinical and histologic study. Journal of Periodontology 80: 776–785
- Barboza, E.P., Stutz, B., Ferreira, V.F. & Carvalho, W. (2010) Guided bone regeneration using nonexpanded polytetrafluoroethylene membranes in preparation for dental implant placements. A report of 420 cases. Implant Dentistry 19: 2–7.
- Ronda M, Rebaudi A, Torelli L, Stacchi C. Expanded vs. dense polytetrafluoroethylene membranes in vertical ridge augmentation around dental implants: a prospective randomized controlled clinical trial. Clinical oral implants research. 2014 Jul;25(7):859-66
- Urban IA, Monje A, Wang HL. Vertical ridge augmentation and soft tissue reconstruction of the anterior atrophic maxillae: a case series. Int J Periodontics Restorative Dent. 2015 Sep 1;35(5):613-23.
- Engler-Hamm D, Cheung WS, Yen A, Stark PC, Griffin T. Ridge preservation using a composite bone graft and a bioabsorbable membrane with and without primary wound closure: a comparative clinical trial. J Periodontol. 2011;82:377–387.
- Carbonell JM, Martín IS, Santos A, Pujol A, Sanz-Moliner JD, Nart J. High-density polytetrafluoroethylene membranes in guided bone and tissue regeneration procedures: a literature review. International journal of oral and maxillofacial surgery. 2014 Jan 1;43(1):75-84
- Jensen SS, Terheyden H. Bone augmentation procedures in localized defects in the alveolar ridge: clinical results with different bone grafts and bone-substitute materials. Int J Oral Maxillofac Implants 2009: 24: 218–236
- Hammerle CH, Jung RE, Yaman D, Lang NP. Ridge augmentation by applying bioresorbable membranes and deproteinized bovine bone mineral: a report of twelve consecutive cases. Clin Oral Implants Res 2008: 19: 19–25
- Urban IA, Lozada JL, Jovanovic SA, Nagursky H, Nagy K. Vertical ridge augmentation with titanium-reinforced, dense-PTFE membranes and a combination of particulated autogenous bone and anorganic bovine bone-derived mineral: a prospective case series in 19 patients. International Journal of Oral & Maxillofacial Implants. 2014 Jan 1;29(1).
- Urban IA, Nagursky H, Lozada JL, Nagy K. Horizontal ridge augmentation with a collagen membrane and a combination of particulated autogenous bone and anorganic bovine bone-derived mineral: a prospective case series in 25 patients. International Journal of Periodontics & Restorative Dentistry. 2013 May 1;33(3).
- Naenni N, Lim HC, Papageorgiou SN, Hammerle CH. Efficacy of lateral bone augmentation prior to implant placement: A systematic review and meta‐analysis. Journal of clinical periodontology. 2019 Jun;46:287-306.
- Feuille F, Knapp CI, Brunsvold MA, Mellonig JT. Clinical and histologic evaluation of bone-replacement grafts in the treatment of localized alveolar ridge defects. Part 1: Mineralized freeze-dried bone allograft. Int J Periodontics Restorative Dent 2003;23:29–35.
- Langer B, Langer L, Sullivan RM. Vertical ridge augmentation procedure using guided bone regeneration, demineralized freeze-dried bone allograft, and miniscrews: 4- to 13-year observations on loaded implants. Int J Periodontics Restorative Dent 2010;30:227–235.
- Beitlitum I, Artzi Z, Nemcovsky CE. Clinical evaluation of particulate allogeneic with and without autogenous bone grafts and resorbable collagen membranes for bone augmentation of atrophic alveolar ridges. Clin Oral Implants Res 2010;21: 1242–1250
- Wood RA, Mealey BL. Histologic comparison of healing after tooth extraction with ridge preservation using mineralized versus demineralized freeze‐dried bone allograft. Journal of periodontology. 2012 Mar 1;83(3):329-36.
About the Authors
 Mehdi Garashi is a Periodontist working for the Ministry of Health of Kuwait and Adjunct Faculty in the Department of Surgical Sciences and Periodontics at Kuwait University College of Dentistry. He is a US-trained dentist and a Diplomate of the American Board of Periodontology. He can be reached at Drmgarashi@gmail.com.
Mehdi Garashi is a Periodontist working for the Ministry of Health of Kuwait and Adjunct Faculty in the Department of Surgical Sciences and Periodontics at Kuwait University College of Dentistry. He is a US-trained dentist and a Diplomate of the American Board of Periodontology. He can be reached at Drmgarashi@gmail.com.
 Dr. Kristen Diaz, is a recent graduate from Nova Southeastern University’s College of Dental Medicine located in Davie, FL. She is currently a first-year resident in Periodontics at Nova Southeastern University, and hopes to work in Miami, FL in the field of Periodontics.
Dr. Kristen Diaz, is a recent graduate from Nova Southeastern University’s College of Dental Medicine located in Davie, FL. She is currently a first-year resident in Periodontics at Nova Southeastern University, and hopes to work in Miami, FL in the field of Periodontics.
 Jon B. Suzuki has a Presidential Appointment as Professor of Microbiology and Immunology in the School of Medicine and Professor of Periodontology and Oral Implantology in the School of Dentistry at Temple University, Philadelphia, PA. USA. He also serves as Chairman and Director of Graduate Periodontology and Oral Implantology at Temple University.
Jon B. Suzuki has a Presidential Appointment as Professor of Microbiology and Immunology in the School of Medicine and Professor of Periodontology and Oral Implantology in the School of Dentistry at Temple University, Philadelphia, PA. USA. He also serves as Chairman and Director of Graduate Periodontology and Oral Implantology at Temple University.
 Diana Bronstein is an associate professor and full time faculty at Nova Southeastern University, Health Profession Division, College of Dental Medicine, (NSU HPD CDM) Periodontology Department since 2010. Dr. Bronstein is licensed in the States of Florida, Ohio, Texas and Virginia and in the EU. degree.
Diana Bronstein is an associate professor and full time faculty at Nova Southeastern University, Health Profession Division, College of Dental Medicine, (NSU HPD CDM) Periodontology Department since 2010. Dr. Bronstein is licensed in the States of Florida, Ohio, Texas and Virginia and in the EU. degree.
RELATED ARTICLE: A Simplified Approach To Lateral Sinus Augmentation
Follow the Oral Health Group on Facebook, Instagram, Twitter and LinkedIn for the latest updates on news, clinical articles, practice management and more!












