Introduction
Patients who are partially edentulous and have a severely resorbed anterior mandibular ridge, either acquired or developmental, often present with significant and challenging problems. These problems include inadequate bone volume for implant supported anterior mandibular fixed prosthetic reconstruction. These deficiencies in bone create significant reconstruction issues for the both the surgical and restorative implant dentist and often require large osseous grafts (Fig. 1). Bone graft healing of this region can sometimes result in significant post-bone grafting resorption for multiple reasons.1,2 Our experience with onlay bone grafting to the anterior mandible has also demonstrated similar significant post bone graft resorption, especially prominent during the first six months (Fig. 2). This type of onlay bone graft volume losses have been reported to range from 25% to 100% and this loss ultimately compromises implant surgery, prosthetic reconstruction and the long-term stability of the implant prosthetic treatment.3,4
FIGURE 1. Intra-oral clinical Photographs: Pre Study Patient AB (No Botox® Patient)
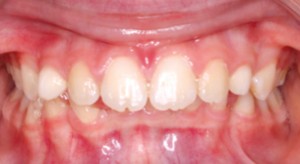
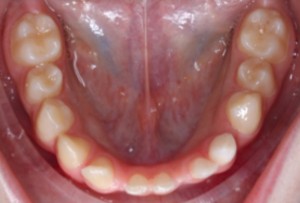
FIGURE 2. Intra-oral post bone graft clinical Photographs: Pre Study Patient AB: demonstrates severe bone resorption following onlay bone grafting (at 3 months and 4 months respectively) (No Botox® Patient).


Peri-oral muscles are one of the main factors responsible for the remodeling progression of bones and dental arches, whether at rest or during stomatognathic system function. Since our bones and teeth react to forces that are applied to them, consequently, strong and well-developed muscles can influence bone strength and shape.5-7 Perioral muscles of the oral stomatognathic system, most notably the mentalis in the chin region can affect form and function. Numerous bone grafting techniques have been described to improve the volume of grafted bone but none have addressed the possible functional aspects of the mentalis and surrounding muscles on bone healing.7-12 The utilization of Botox® therapy has never been published as a possible adjunctive and/or ancillary procedure to reduce post bone graft resorption.
This preliminary study will present a potential benefit of Botox® (Allergan Inc., Botulinum toxin type A) (BTA) for reduction of post-bone graft resorption with the primary goal to improve overall bone quantity and quality. Patients with inadequate bone volume for implant placement that required anterior mandibular onlay block bone grafts from the ramus were included in this study. The aim of this study was to assess the clinical outcomes of post bone graft healing associated with oral myofunctional alterations of anterior mandibular bone grafts in conjunction with Botox® therapy.
Methods and Materials Patients
In 2008, our group elected to proceed with an observational study of Botox® (BTA) injections of select patients who required significant anterior mandibular onlay bone grafting for implant reconstruction of partially edentulous spaces. All patients included in this study had severe anterior mandibular atrophic bone, either acquired or developmental, in the (antero-posterior) transverse dimension. All patients were healthy ASA I patients and non-smokers. Twelve patients, over a period of seven years, where selected to participate in this preliminary study. All surgical treatment was completed by two oral and maxillofacial surgeons. These patients were scheduled for eventual implant prosthetic reconstruction and presented with inadequate bone volume in the anterior mandible for implant placement (Fig. 3). A clinical and radiographic examination was performed to evaluate the bone graft donor site and recipient sites. The choice of donor site was the lateral posterior body/ramus of the mandible. Cone beam Computed Tomography (CBCT) imaging was utilized with the ICAT (Imaging Science International) system to evaluate the donor and recipient sites, preoperatively and postoperatively after at least four months of healing and at the time of implant placement. The preoperative CBCT was utilized for mapping out the inferior alveolar canal in the region of the harvest site (Fig. 4).
FIGURE 3. Intra-oral clinical Photograph: Patient #1.

FIGURE 4. Patient #2: Pre bone graft radiographic evaluation (ICAT CBCT)

Surgical Technique
All patients were treated under intravenous sedation or general anesthesia. Pre-operative prophylactic antibiotics included either Ancef (cefazolin) 1 gm IV or Clindamycin 600 mg IV prior to surgical incisions. The donor and receptor sites received mandibular blocks and infiltration with local anaesthetic (Marcaine 0.5% with 1: 200,000 epinephrine) under either intravenous sedation or general anaesthesia, as appropriate. The proposed recipient site for the graft was exposed prior to graft harvest in all cases. In this manner, the dimensions and morphology of the bony defect were measured, and minimal time elapsed between graft harvest and placement. The surgical techniques for procuring bone grafts from the ramus areas have been described by Misch.10 The lateral part of the ramus was exposed, and a mainly cortical bone graft was harvested from the lateral cortex of the ramus (Fig. 5). At the recipient site, a midline crestal incision with or without a releasing incision was utilized to expose the anterior mandible and maintain a blood supply. A full-thickness buccal and crestal flap was elevated to expose the reconstruction area (Fig. 6). There was minimal elevation of the lingual aspect of the mucosal flap. The recipient sites were re-contoured and perforated, with a number 6 or 8 round bur when necessary to improve bone-to-graft contact improved blood supply to the donor graft. The block grafts were fixed with a 1.5 mm KLS Martin osteosynthesis screws at bone level (Fig. 7). These screws were placed in a bicortical manner to provide absolute graft to recipient site rigidity. Prior to primary closure, two buccal sites were measured on or beside the screw head. The buccal/lingual width of the graft and recipient site was recorded for the two sites with the use of Castroviejo Bone Calipers and/or CBCT (T1) (Fig. 8). The two sites were measured along the long axis of the screw. A periosteoplasty was performed on the buccal subperiosteal mucosal flap to allow for a mucosal advancement over the graft and a primary tension-free adaptation of the wound margins. Either Puros® Dermis Allograft Tissue Matrix (Zimmer Dental) or Geistlich Bio-gide® resorbable bilayer collagen membrane (Hansamed Limited) membranes where utilized over the block grafts depending on clinical soft tissue needs. In addition, Geistlich Bioss® xenograft material was utilized to fill any small osseous voids around the ramus onlay block grafts (Figs. 9 & 10). All sutures utilized to close the sites were Ethicon® Prolene* (polypropylene) blue monofilament, non-absorbable and all sites were allowed to heal for at least four months prior to stage I implant surgery (Fig. 11). After completion of the closure, a subcutaneous/intra-muscular injection of 10 units of Botox® was completed bilaterally in each of the mentalis muscles (20 units total) (Fig. 12). Vacuum-formed Essix® retainers with incorporated provisional prosthetic teeth were utilized post grafting and were adjusted to avoid soft tissue contact. Antibiotic coverage was continued for one week postoperatively. Analgesics, including appropriate narcotic and NSAID were prescribed to manage postoperative pain.
FIGURE 5. Patient #4: Intra-operative photo demonstrates harvesting block graft right posterior mandible/ramus.

FIGURE 6. Patient #2: Intra-operative photo demonstrates severe atrophic mandible.

FIGURE 7. Patient #2: Intra-operative photo demonstrates ramus onlay block graft fixation utilizing 1.5 mm KLS Martin Osseous Screws.

FIGURE 8. Patient #3: Intra-operative Castroviejo Bone Caliper technique for measuring grafted sites.

FIGURE 9. Patient #2: Intra-operative photos demonstrate placement of Bio-gide® collagen membrane prior to primary closure.

FIGURE 10. Patient #3: Intra-operative photos demonstrate placement of Puros® Dermis Allograft prior to primary closure.

FIGURE 11. Patient #2: Intra-operative photo demonstrates primary closure utilizing 4-0 Prolene Sutures (Graft/BTA).

FIGURE 12. Diagram and Extra-oral photo demonstrates bilateral mentalis muscles (blue dots) and injection sites for Botox.



After adequate healing period of two to three weeks all sutures were removed (Fig. 13). During the post bone graft healing phase possible complications were evaluated. These included donor incision dehiscence, nerve injury (paresthesia), donor site infection, complications associated with graft harvest and complaints of altered sensation of teeth and mucosa proximal to the graft and any facial esthetic concerns.
FIGURE 13. Patient #2: 3 weeks post bone graft and just after suture removal (Graft/BTA).

At least four months of bone graft healing was allowed to pass prior to implant Stage I surgery (Fig. 14). At the time the patients were scheduled for implant surgery intra-operative evaluation of the graft site at the same two screw head sites was completed. The same crestal incision was followed by mucosal flap reflection for implant placement and bone resorption was calculated based on the measurements at the fixation screw heads (Fig. 15). The actual measurement of the bone width was completed on both the CBCT and with the use of intra-operative Castroviejo Bone Calipers. The two sites were measured, at time of re-entry (T2) along the long axis of the screw, which duplicated the same position at the time of initial bone grafting (T1) (Fig. 16). All intra-operative bone sites width was evaluated as described above and were compared to the pre-operative measurement (T1). Percentage of bone loss was determined from the top or beside the head of the screw to the new remodeled mandibular buccal cortex as outlined above. This measurement (T2) was used to calculate the percentage of bone loss from the original measurement (T1), which produced the mean effective bone volume loss percentage (EBVL).
FIGURE 14. Patient #2: 4 months post bone graft to anterior mandible with concurrent Botox ® therapy (Graft/BTA).

FIGURE 15. Patient #2: 4 months post bone graft to anterior mandible with Botox ® therapy (Graft/BTA).


FIGURE 16. Patient #2: Measurement of Bone width 4 months post bone graft (Graft/BTA).

During the intra-operative implant surgery phase clinical evaluation included incorporation and resorption following graft healing, bone quality of the healed graft and implant placement in the grafted sites. The morphology was grossly assessed as cortical or corticocancellous. Bone quality was assessed during drilling of the implant osteotomy sites. The bone quality classification ranking of types one through four, as proposed by Lekholm and Zarb14 and described by Misch15 was recorded.
Results
Twelve procedures, as described above, to reconstruct anterior mandibular bone defects were performed on 12 patients (eight females, four males). The mean age was 22.2 years (range 18-32 years). Ten patients underwent general anesthesia and two underwent intravenous sedation. Follow-up period after the bone graft/BTA therapy ranged from four months to seven months. This coincided with the time of implant surgery.
There were no significant complications either during or following the grafting/Botox® treatment. Two patients complained of tenderness over the screw heads at the time of implant surgery. There were no dehiscence or post-operative infections. Neurosensory function was assessed and was noted normal for all patients. There was no incision line dehiscence, sensory deficits at either the donor site or recipient site. No patients complained about reduced mentalis muscle function or changes in facial expression. There was minimal patient concern for altered facial function related to the mentalis and peri-oral muscles. All 12 onlay blocks grafts sites had excellent consolidation of the bone and all grafts were incorporated with the recipient site. Following osteosynthesis screw removal all treatment planned implants sites had successful implant placement at the time of re-entry.
The mean effective bone volume loss (EBVL) was 14.4% after bone grafting surgery, measured at the time of graft exposure and implant placement. The bone quality at the implant sites was more often graded as quality Type 1 to 2 for all sites. At the time of re-entry, there was a notable volume of excellent bone present as compared to our previous experience without BTA. There was reduced and significant clinical difference of resorption volume as compared to our experience with no Botox® Therapy. The previous no BTA (no Botox®) procedures demonstrated resorption rates ranging up to 50% based on our subjective clinical experience. The result of this study revealed that the combination of onlay grafting and BTA resulted in bone resorption rate that ranged from 10.1% to 16.8% (Table 1).
TABLE 1. Effective Bone Loss (with BTA) at time of implant surgery.

Discussion
In 2008, we noted significant volume of post bone graft resorption, despite cortical block augmentation with the use of resorbable membranes for partially edentulous anterior mandible onlay block grafting. Loss of augmented bone volume was noted to be quite aggressive in the immediate short term and resulted in both clinical and radiographic disappointment. These findings were consistent with other authors.16,17 Our group hypothesized that the aggressive bone resorption associated with anterior mandible onlay bone graft loss could be associated with and related to peri-oral myofunctional stress and load. The mentalis muscle had been implicated for the bone volume loss during the early post-bone graft healing phase with other authors.7,17 It was at that time two oral and maxillofacial surgeons elected to inject Botox® into the mentalis muscle at the time of onlay bone grafting of the anterior anterior mandible.
Botulinum Toxin Type A (BTA)
The first application of Botox® as medication occurred in the 1960’s for the treatment of certain neurological disorders. Subsequently, BTA was finally approved in 1989 by the FDA (US Food and Drug Administration) at which time it was indicated for treatment of some eye disorders caused by muscular dysfunction. Botulinum Toxin Type A (BTA) is a neurotoxin derived from bacterium clostridium botulinum. BTA inhibits the release of acetylcholine (ACH), a neurotransmitter responsible for the activation of muscle contraction and glandular secretion, and its administration results in reduction of tone in the injected muscle for three to four months18 (Fig. 17). BTA has a long history of many medical therapeutic uses such as the management of muscle spasms with cerebral palsy, cervical dystonia, hyperhidrosis, strabismus and blepharospasms. Since then BTA, and the seven other forms of the Botulinum toxin, have been continuously researched and tested.
In 2001, Botox® was first approved by Health Canada for cosmetic treatment of hyperdynamic facial lines of the face, but BTA has now been increasingly used in dentistry as well due to its therapeutic uses in treatment of certain maxillofacial conditions, such as oro-facial dystonia and myofascial pain. In Alberta, oral and maxillofacial surgeons have been utilizing BTA for treatment of both esthetic and therapeutic reason since 2003. Botulinum Toxin Type A has been used to treat the following maxillofacial and dental conditions; Temporomandibular joints disorders, bruxism, oro-mandibular dystonia, muscles of mastication spasm, pathologic clenching, gummy smile, masseteric hypertrophy, sialorrhea, retraining of muscles during orthodontic treatment and prophylactically to diagnose dental pain.19 The use of BTA is a minimally invasive procedure and is showing quite promising results in management of muscle-generated dental diseases like Temporomandibular disorders, bruxism, clenching, masseter hypertrophy and used to treat functional or esthetic dental conditions like deep nasolabial folds, radial lip lines, high lip line and black triangles between teeth.20
FIGURE 17. A nerve with an enlarged view of the nerve endings, receptors on the muscle, and acetylcholine either released or blocked by Botox®.

Peri-oral Muscle Anatomy
The anterior mandible anatomy and perioral muscles function in harmony with the mentalis muscles (MM). The mentalis is a muscle located directly below the lower lip, near the chin tip. It is responsible for raising the lower lip.21 The MM is the only elevator of the lower lip and the chin, and it provides the major vertical support for the lower lip.
The morphology of the mentalis muscle is complex as it has very special and symbiotic relationship to other peri-oral muscles, especially in relation to the orbicularis oris muscle and the incisivus labii inferioris muscle (ILI) as studied by Hur.22 The medial fibers of both MMs descended anteromedially and crossed together, forming a dome-shaped chin prominence. The majority of the lateral fibers of the MM descended inferomedially. The upper fibers of the MM were short and run horizontally, whereas the lower fibers were long and descended inferomedially or vertically. The upper fibers of the MM were intermingled with the inferior margin of the orbicularis oris muscle. The originating muscle fibers of the ILI were intermingled with the upper lateral MM.22 The origin is from the Incisive fossa of the mandible, its fibers arise from the incisive fossa of the mandible, then course vertically downward to insert into dermis of the chin skin. Sometimes at this origin, the mentalis muscle fibers have been found to insert into the periosteum of the alveolar bone at the apex of the mandibular incisors or very close to the superior aspect of the residual ridge.23 The insertion of the mentalis muscles is into the dermis of the chin region. The mentalis muscle has two actions; protrusion of the lower lip and elevation and wrinkling of the skin of the chin.
The results of this anatomical structure has constituted a new anatomical knowledge and respect for the MM and its complex interaction and function with adjacent muscles and possible effects on teeth overlying native or grafted bone.4,7,23-25 Reduction of this complex interaction may explain the results of reduced bone resorption when anterior mandibular grafting is combined with BTA.
Anterior Mandibular Bone Grafting / Peri-Oral Muscle Function
Reconstruction of the anterior atrophic mandible by means of onlay bone grafting using an intraoral autologous bone graft has been the preferred procedure when there is inadequate bone volume to allow for dental implant placement. The use of autologous bone grafts with osseointegrated implants was originally discussed by Brånemark26 and it is now a well-accepted procedure in oral and maxillofacial rehabilitation.15 Bone grafting is an essential aspect of treatment required for the dental implant reconstruction. Unfortunately, bone resorption has long been a serious problem following grafting procedures, especially in the anterior mandibular region.1,2,16,17
A myriad of factors have been reported to possibly have a direct effect on the bone healing cascade, including and not limited to surgical technique, embryologic origin of the donor bone, quality of tissues, blood supply, mechanical stress, types of autologous and non-autologous graft materials, stability of grafting materials, membranes, systemic factors, growth factors, regulatory proteins and external forces that direct the bone healing cascade.16,17,27 Post graft remodeling and resorption is most significant in the first six months.28,29,32 In our previous experience, we noted significant bone loss but in this study with the use of BTA the bone stability improved both clinically and radiographically with mean effective bone loss of approximately 14.4%.
Botox® is not new to dentistry. In regard to dental implant reconstruction there are numerous articles that describe treatment of excessive occlusal load related to implant healing. The overloading of the muscles of mastication can prevent or impede osseointegration of implants and/or fracture callus formation.19,20 The muscular relaxation achieved with botulinum toxin type injections to the masticatory muscles can be therapeutically beneficial by creating a better environment for implants osseointegration and fracture healing in a more stable environment.30,33
The mentalis muscle, which is a midline bilateral muscle of the chin region can influence both bone and tooth position. Tang7 analyzed chin augmentation bone grafts harvested from the mandibular angle and noted undesirable levels of bone resorption in 73.9% of cases with, complete resorption in five cases.22 Kayikvioglu20 conducted a small study to prospectively evaluate the use of botulinum toxin type A as an adjunct to zygomatic fracture fixation surgery, in an attempt to reduce the number of fixation sites and to prevent dislocation of the zygomatic bone. Five male patients with zygomatic bone fractures were injected with 100 U of botulinum toxin type A into the masseter muscle of the fractured side. Patients were then operated on 12 to 48 hours after the injection and EMG confirmation of muscle denervation. The temporary paralysis of the masseter muscles allowed for fewer miniplate and/or microplate insertions in all patients, and resulted in no complications related to either the botulinum toxin injections or surgical procedures. These authors have hypothesized that the treatment of excessive muscle function could reduce bone resorption. This is consistent with our results with anterior mandibular bone grafting and BTA.
In 1989, Whitaker35 presented the theory of the biological boundary, accentuating that the soft tissue envelope has a genetically predetermined shape, which is programmed to remain constant. This concept of biological boundary was truly an extension of Moss’s functional matrix theory.36 Moss’s theory presented the concept that as the craniofacial skeleton grows, the soft tissue environment shapes its morphology. Whitaker applied Moss’s functional matrix theory to bone grafting. He hypothesized that onlay grafts violate the body’s natural soft tissue boundaries, which, in turn, elicits a homeostatic response to maintain the boundary and resorb the graft. Furthermore, these post bone graft changes are a further extension of Wolff’s law, which states, “Remodeling of bone occurs in response to physical stresses”. In essence, a bone’s form follows its function.28
Onlay grafts within a tight tissue envelope will experience forces that are different from grafts within a loose soft tissue envelope. Onlay grafts that underlie muscle, a relatively tight tissue envelope, withstand a significant compressive force. For example, onlay grafts placed underneath the temporalis muscle invariably show significant resorption. Therefore, by relaxing these muscles, like the mentalis, it appears based on our study that there is less bone resorption occurring over the short term. This results with BTA may also support the possibility of “shielding hypothesis” during the early healing phase by diminution of overlying soft issue tensile forces that which lead to improved and maintained bone graft volume.28,33,37 Based on our findings there appears to be a significant shielding occurring with BTA and the associated affect of the mentalis musculature associated and symbiotic muscles. The shielding appears to be beneficial in the early healing phase thus reducing early aggressive bone resorption that has been previously experienced and reported.34,38 Although this is only a short term effect it appears to be critical in the early initial healing of the bone graft site. The effect of myofunctional forces does appear to effect bone resorption and stability. Further research needs to be completed, with random, double-blind studies or even split mouth studies to better understand this relationship of muscle function to bone graft healing along with BTA.
Conclusions
Implant surgeons and restorative dentists are continually faced with inadequate bone volumes prior to implant reconstruction. An adequate osseous foundation is the corner stone for successful implant osseointegration and dental rehabilitation. Establishing architecturally stable bone substructure is just one critical factor that is absolutely imperative for long-term success with dental implant rehabilitation. Autogenous bone grafts harvested from the mandibular ramus offer several advantages in the reconstruction of alveolar ridges for implant placement but can significantly resorb and remodel after augmentation procedures. There are a complex array of factors influencing the remodeling of bone under stress and load that has been traditionally been referred to as Wolff’s Law. It is essential that dentists have a clear understanding of bone graft physiology and the myofunctional relationship of bone and muscle. The preliminary result of this study supports that concomitant use of Botox® at the time of onlay block graft procedures. Botox® has demonstrated an advantage of reduced post bone graft resorption without additional morbidity or complications. Botox® demonstrated reduced mentalis and peri-oral muscle function, through shielding, thus exhibiting reduced bone resorption and improved maintenance of graft volume in the short term healing period allowing for more reliable primary implant placement after appropriate graft maturation. These preliminary findings will encourage further research and potentially longer, more controlled multi-center studies contributing to improved care for these difficult dental reconstruction cases. OH
Kevin E. Lung, BSc, DDS, MSc, FRCD, Kingsway Oral & Maxillofacial Surgery, Edmonton, Alberta Email: k.lung@kingswayos.com.
Walter Dobrovolsky, BSc, DDS, FRCD, Kingsway Oral & Maxillofacial Surgery, Edmonton, Alberta. E-mail: dobs@kingswayos.com.
Keith H. Compton, BSc, DDS, MSc., Prosthodontists, Vivid Prosthodontics, Edmonton, Alberta. E-mail: dr.compton@vividdental.ca.
Cornell K. Lee, DDS, MDSc, FRCD, Dental Design Concepts, Edmonton, Alberta. E-mail: drclee@drclee.ca.
Ian W. McKee, BSc, DDS, MSc, FRCD, Signature Orthodontics, Edmonton, Alberta. Email: drimckee@signatureorthodontics.com.
Oral Health welcomes this original article.
References:
1. Chiapasco M, Casentini P, Zaniboni M. (2009) Bone augmentation procedures in implant dentistry. The International Journal of Oral & Maxillofacial Implants, Vol.24, Suppl, (October 2009),
pp. 237-259.
2. Milinkovic I, Cordaro L. (2014) Are there specific indications for the different alveolar bone augmentation procedures for implant placement? A systematic review. Int J Oral Maxillofac Surg. 2014 May;43(5):606-25.
3. Bernstein S, Cooke J, Fotek P, Wang HL. (2006) Vertical bone augmentation: where are we now? Implant Dentistry, Vol.15, No.3, (September 2006), pp. 219-228.
4. Breine U, Brånemark P-I. (1980) Reconstruction of alveolar jaw bone. An experimental and clinical study of immediate and preformed autologous bone grafts in combination with osseointegrated implants. Scan J Plast Reconstr Surg, 1980;14:23–48.
5.de Souza DR, Semeghini TA, Kroll LB, Berzin F. (2008) Oral Myofunctional and electromyographic evaluation of the orbicularis oris and mentalis muscles in patients with Class II/1 malocclusion submitted to first premolars extraction. J Appl Oral Sci. 2008 June; 16(3): 226–231.
6. Shi L., Zhang ZY, Tan XJ. (2013) Severe bone resorption in expanded polytetrafluoroethylene chin augmentation. J Craniofac Surg. 2013 Sep;24(5):1711-2.
7. Tang X, Gui L, Zhang Z. (2009) Analysis of chin augmentation with autologous bone grafts harvested from the mandibular angle. Aesthet Surg J. 2009 Jan-Feb;29(1):2-5.
8. Jensen OT. (1994) Guided bone graft augmentation. In: Buser D, Dahlin C, Schenk RK (eds). Guided Bone Regeneration. Chicago: Quintessence, 1994:235–264.
9. Chiapasco M, Zaniboni M. (2011) Failures in jaw reconstructive surgery with autogenous onlay bone grafts for pre-implant purposes: incidence, prevention and management of complications. Oral Maxillofac Surg Clin North Am. 2011 Feb;23(1):1-15,
10. Misch CM. (1996) Ridge augmentation using mandibular ramus bone grafts for the placement of dental implants: Presentation of a technique. Pract Periodontics Aesthet Dent 1996;8:127–135.
11. Male AJ, Gasser J, Fonseca RJ, Nelson J. (1983) Comparison of onlay autologous and allogenic bone grafts to the maxilla in primates. J Oral Maxillofac Surg 1983;42:487–499.
12. Buser D, Bragger U, Lang NP, Nyman S. (1990) Regeneration and enlargement of jaw bone using guided tissue regeneration. Clin Oral Implants Res 1990;1:22–32.
13. Swart JG, Allard RH: (1985) Subperiosteal onlay augmentation of the mandible: a clinical and radiographic survey. J Oral Maxillofaca. Surg. 1985: Mar., 43(3): 183-187.
14. Lekholm U, Zarb GA. (1985) Patient selection and preparation. Tissue integrated prostheses: osseointegration in clinical dentistry. Branemark PI, Zarb GA, Albrektsson T, editor. Chicago: Quintessence Publishing Company; 1985. p. 199–209.
15. Misch CE, Judy KW. (1987) Classification of partially edentulous arches for implant dentistry. Int J Oral Implantol. 1987;4(2):7-13.
16. Aloy-Prósper A, Peñarrocha-Oltra D, Peñarrocha-Diago M, Peñarrocha-Diago M. (2015) The outcome of intraoral onlay block bone grafts on alveolar ridge augmentations: a systematic review. Med Oral Patol Oral Cir Bucal. 2015 Mar 1;20(2):e251-8.
17. Proussaefs P, Lozada J. (2003) The use of resorbable collagen membrane in conjunction with autogenous bone graft and inorganic bovine mineral for buccal/labial alveolar ridge augmentation: a pilot study. J Prosthet Dent. 2003 Dec;90(6):530-8.
18. Botox® onabotulinumtoxinA Product Monograph, Allergan Canada Inc. 2016.
19. Nayyar P, Kumar P, Nayyar PV, Singh A. (2014) Botox: Broadening the horizon of dentistry. J Clin Diagn Res. 2014 Dec; 8(12); 2014 Dec.
20. Azam A, Manchanda S, Thotapalli S, Kotha SB. (2015) Botox Therapy in Dentistry: A Review. J Int Oral Health. 2015;7(Suppl 2):103-5.
21. Kane M, Sattler G. (20130 Illustrated Guide to aesthetic Botulinum Toxin Injections, United Kingdom, Quintessence Publishing, 2013, p. 88-91. Collins T, Small S, Shepard N, Buser D, Parel S. Sinus-floor elevations and the status of membranes. Int J Oral Maxillofac Implants 1994;9(suppl):85–96.
22. Hur MS, Kim HJ, Choi BY, Hu KS, Kim HJ, Lee KS. (2013) Morphology of the mentalis muscle and its relationship with the orbicularis oris and incisivus labii inferioris muscles. J Craniofac Surg. 2013 Mar;24(2):602-4.
23. Harradine NW, Kirschen RH. (1983) Lip and mentalis activity and its influence on incisor position—a quantitative electromyographic study.Br J Orthod. 1983 Jul;10(3):114-27.
24. Zide BM, McCarthy J. (1989) The mentalis muscle: an essential component of chin and lower lip position. Plast Reconstr Surg. 1989 Mar;83(3):413-20.
25. Zarb GA, Hobkirk J, Eckert S. Jacob RF. (2013) Prosthodontic Treatment for Edentulous Patients: Complete Dentures and Implant Supported Prostheses. 13th Edition, Mosby, 2013 p. 86-88
26. Brånemark P-I, Lindstrom J, Hallen O, Briene U, Jeppson P-H, Ohman A. (1975) Reconstruction of the defective mandible. Scand J Plast Reconstr Surg 1975;9:116–128.
27. Tolman DE. (1995) Reconstructive procedures with endosseous implants in grafted bone: A review of the literature. Int J Oral Maxillofac Implants 1995;10:275–294.
28. Oppenheimer AJ, Tong L, M.D., and Buchman SR. (2008) Craniofacial Bone Grafting: Wolff’s Law Revisited. Craniomaxillofac Trauma Reconstr. 2008 Nov; 1(1): 49–61.
29. Nishimura K, Itoh T, Takaki K, (2003) Periodontal parameters of osseointegrated dental implants: A four-year controlled follow-up study. Clin Oral Implants Res. 1997;8(4):272–78.
30. Kayikvioglu A, Erk Y, Mavif E. (2003) Botulinum toxin in the treatment of zygomatic fractures. Plast Reconstr Surg. 2003;111(1):341–46.
31. Zide BM, McCarthy J. (1989) The mentalis muscle: an essential component of chin and lower lip position. Plast Reconstr Surg. 1989 Mar;83(3):413-20.
32. Pikos MA. (2005) Atrophic posterior maxilla and mandible: alveolar ridge reconstruction with mandibular block autografts. Alpha Omegan. 2005 Oct;98(3):34-45.
33. Kumar P1, Khattar A, Goel R, Kumar A. (2013) Role of botox in efficient muscle relaxation and treatment outcome: an overview. Ann Med Health Sci Res. 2013 Jan;3(1):131
34. Cordaro L, Amadé DS, Cordaro M. (2002) Clinical results of alveolar ridge augmentation with mandibular block bone grafts in partially edentulous patients prior to implant placement. Clin Oral Implants Res. 2002 Feb;13(1):103-11.
35. Whitaker LA. (1989) Biological boundaries: a concept in facial skeletal restructuring. Clin Plast Surg. 1989 Jan;16(1):1-10.
36. Moss ML, Rankow RM. (1968) The Role of the Functional Matrix in Mandibular Growth. The Angle Orthodontist: April 1968, Vol. 38, No. 2, pp. 95-103.
37. Goldstein J, Mase C, Newman M H. (1993) Fixed membranous bone graft survival after recipient bed alteration. Plast Reconstr Surg. 1993;91(4):589–596.
38. Peñarrocha-Diago M, Aloy-Prósper A, Peñarrocha-Oltra D, Guirado JL, Peñarrocha-Diago M. (2013) Localized lateral alveolar ridge augmentation with block bone grafts: simultaneous versus delayed implant placement: a clinical and radiographic retrospective study. Int J Oral Maxillofac Implants. 2013;28:846-53.
Follow the Oral Health Group on Facebook, Instagram, Twitter and LinkedIn for the latest updates on news, clinical articles, practice management and more!












