Introduction
Extractions & Ridge Atrophy
Alveolar ridge atrophy and morphological changes subsequent to exodontia have been extensively investigated and the pathophysiology is now well understood.1 Loss of bone support in the alveolar ridges posses a great challenge for dental restorative procedures. Treatments such as removable dental prosthesis devices (RDPDs) rely on the ridge as the primary source of support. Fixed dental prostheses require adequate ridge height for aesthetic, functional and adequate oral hygiene reasons. Sufficient alveolar bone density and morphology is a prerequisite for implant placement and success.2 Bone regenerative techniques facilitate preservation of the alveolar ridge and have been shown to improve the outcomes of several dental treatments. To date, a plethora of studies have evaluated the components of guided bone regeneration (GBR) procedures.
This article summarizes the biological basis and components of GBR and provides evidence-based recommendations for consideration among dental clinicians.
Background
Bone Healing & Wound Repair
The healing of bone is unlike other tissues in its ability to regenerate itself rather then form a fibroblastic scar. This healing process is divided into primary and secondary healing. In general, both processes begin with the formation of a hematoma and result in the remodeling of woven bone into the stronger lamellar structure. In secondary healing, which occurs when the edges of the bone are not in close proximity (>1mm), endochondral ossification induces the intermediate formation of a cartilaginous callus model.3 The ability and rate of this healing process is dependent on vascular perfusion, mechanical stability, space for the hard tissue formation, the presence of stem cells and site isolation via close approximation of the soft tissue edges.4
Similar to bone healing, wound repair involving the gingiva and mucosa, is divided into overlapping phases. Initially, hemostasis produces a coagulum and activates platelets. These activated platelets release cytokines that attract inflammatory cells to the site. Neutrophils accumulate during the acute phase; they eliminate pathogens, remove damaged cells and secrete cytokines that induce the proliferation of macrophages. Macrophages perform many functions similar to those of neutrophils, in addition they release cytokines that stimulate the differentiation of mesenchymal cells into fibroblast and promote angiogenesis. These changes mark the transition into the proliferative phase during which, collagen deposition, wound contraction, and angiogenesis/vascularization produce granulation tissue. Distinct from bone healing, an epithelialization process of re-attachment of opposing ends of epithelium occurs during the healing of a laceration or surgical wound. Eventually, the maturation phase results in replacement of granulation tissue and resolution of the wound.4 Primary intention wound healing occurs in the absence of a gap between the edges of the laceration; however if a large space remains during healing, the proliferative phase is prolonged resulting in increased collagen deposition and wound contraction which generally produces a scar, a process known as secondary intention healing.3
Subsequent to the extraction of a tooth, the alveolar socket undergoes healing via secondary intention. The process of wound healing previously described occurs in conjunction with that of secondary bone healing.1,5 Osteoblasts and osteoclasts facilitate the regeneration/remodeling of the bony matrix while epithelialization attempts to isolate the site from the external environment.4
The simultaneous occurrence of these healing events manifests as a race between the epithelial isolation and the osteoblastic bone regeneration. Epithelial cells often win this race due to faster apical migration than other reparative cells (e.g. Osteoblasts). This results in a reduced height of the alveolar bone level creating a crater-like defect and decreased bone density in the affected ridge area.4,5
Current Preservation Techniques
Guided Bone regeneration is a process used to prevent and restore the resorption of the alveolar ridge associated with tooth loss. As discussed previously, the regeneration of bone depends upon several factors and requirements (vascularity, stability, space, site isolation and stem cells). Providing these factors and meeting the requirements, in turn, creates an ideal environment for the regeneration of bone.
Bone grafts have been thoroughly studied and used to meet these requirements; grafts provide space and stability for vascularization while either promoting or providing the cellular components needed for bone formation. A graft is characterized by it mechanism as osteoconductive, osteoinductive or osteogenesis. Osteoconductive grafts function as a scaffold for native osteoprogenator cells to form bone. Grafts that induce the activation of osteoblast via protein mediators function via osteoinduction. Osteogenesis bone grafts contain viable osteoblast that act in accord with the native cells to form new bone.4,6
Demineralized freeze-dried bone allograft (DFDBA):
For several years, the use of DFDBA proved helpful in GBR by reducing the amount of alveolar ridge bone loss when compared to sockets without placement of grafting material.7,8,9 DFDBA acts as a scaffold by maintaining the extraction space and stabilizing the site ultimately permitting angiogenesis. Originally, these allografts were thought to contain adequate BMP proteins to induce the formation of bone; however since their introduction numerous studies have indicated that the osteoinductive properties are not always intrinsic to the material and depend on several factors, such as the demographic of the donor.10,11
Allografts of tooth structure:
Recently, the use of extracted teeth has been studied and found to have an equal to slightly greater efficacy in site preservation as DFDBA.12 Initially autogenous tooth structure was obtained from a harvesting site during the initial procedure, processed chair-side and used immediately as it was thought to minimize the risk of graft rejection. However, this procedure requires ample time and the quality of the graft is dependent on the condition of the patients tooth to be extracted.13 More recent studies have evaluated the use of tooth structure allografts (obtained from another human source) in place of the autografts. These have so far proven equivalent or slightly more successful than the tooth structure autografts, suggesting a new faster, more reliable option for clinicians.12,13 One must keep in mind that use of a graft material from extrinsic source poses the risk of an adverse immunogenic reaction; this risk should be evaluated when considering graft options for each case.
Autogenous bone:
An ideal graft material should be biocompatible, easy to use, and reliable. Additionally it should have osteoconductive, osteoinductive and ostegenesis properties. Autogenous bone possesses these qualities making it the gold standard in GBR.14,15 Because this graft material is directly harvested from the patient, the risk of immune rejected is essentially eliminated. The surgeon will typically harvest from one of several sites including the mandibular ramus or symphysis, the coronoid process, the extraction socket area, the maxillary tuberosity or if more bone is indicated extra-oral sites may be used. The decision on which site to harvest from is based on many factors specific to each case.14,15,16,17
Barrier Membranes:
Referring back to the discussion on socket healing, excluding the epithelial and connective tissues from the bony defects permits hard tissue formation via osteogenic cell function.19 For decades, barrier membranes have enabled this exclusive process. Optimally a barrier membrane should preserve the bony defect space by occluding soft tissue cells, it should be biocompatible with desirable handling properties and should either be resorbable or be easy to remove.4 Many different materials have been introduced and more research is underway in search of developing an “ideal” membrane. At present, two general types of membranes exist, non-resorbable and resorbable both with their own advantages and disadvantages.
Non-resorbable membranes are biocompatible and offer excellent mechanical stability, as they may be Titanium reinforced. Additionally, they generally remain in place for adequate time for complete resolution to occur. Of course, these membranes require an additional surgical procedure for removal and are associated with an increased risk of infection if exposure occurs prematurely.20 The elimination of a second surgical procedure and hence decreased patient morbidity have caused resorbable membranes to gain attention among clinicians and researchers. However, the inability to control the degradation rate of the membranes limits the duration and quality of the socket healing process.21 Several studies comparing the use of resorbable versus non-resorbable membranes in GBR have found no significant differences in clinical outcomes for either.22,23,24 The decision of which type of membrane to used should be based on clinicians preference and specific patient characteristics (immunocompetency status, religion, etc.
Conclusion
An immense amount of research has been conducted to elucidate the physiology of GBR and towards the development of biological materials for enhancement of treatment strategies. To date, meta-analyses and systematic reviews of literature have demonstrated the effectiveness of GBR procedures in preservation of the alveolar ridge.25,26 As briefly illustrated in this review, a tremendous amount of variation regarding the ways to achieve GBR exists. Although options and alternatives for treatment protocols enhance specific patient outcomes, they also may leave clinicians uncertain of the optimal treatment; in turn resulting in avoidance of the protocol altogether. In the next section, evidence-based recommendations for GBR protocols are provided. The astute reader should keep in mind that each individual may require some variation from these recommendations and should always use clinical judgment in conjunction with them.27-29
Clinicians Guide
The decision to use GBR in a patient undergoing exodontia should be based on several patient-specific considerations such as the location of the extraction, the restorative plan for the site as well as the patients demographic and preferences. If the area is to remain unrestored, e.g. certain third molar sites, minimal benefit may be achieved from an adjunctive procedure such as GBR. The patient may not be a candidate for such a procedure due to an immunocompromised status, financial constraints or personal preferences (religious beliefs, etc.). In situations for which GBR would provide maximum benefits the decision of which material to use becomes important. If the patient intends to have the missing tooth site restored by an implant within a year post extraction, GBR is indicated. Pontic areas, especially in the esthetic zone, warrant ridge preservation procedures to improve hygiene and appearance. Removable dentures require sufficient ridges to rest on, if they are even partially tissue born. It is an intended side effect of GBR that the covering tissues often improve in keratinization and thickness.30-34
CASE REPORT
Extraction and Immediate Implant with Delayed Loading Protocol.
Clinical Presentation
A 77-year-old Caucasian female non-smoker with history of Hypothyroidism presented with a chief complaint of “My Dentist referred me to replace this broken tooth with a dental implant”. Initial clinical examination revealed fractured tooth #4 surrounded with heavily restored posterior teeth. According to the patient, the tooth fractured recently and upon close inspection, deep subgingival decay was detected on the remaining root. The patient had fair oral hygiene with localized mild gingival erythema, adequate mesio-distal and apico-coronal restorative space, and there was no contraindication to dental implant surgery (Fig. 1). Bitewing radiograph revealed fractured tooth #4 with a radiolucency approaching the pulp. In addition, generalized mild horizontal bone loss involving the upper right posterior teeth was evident on the bitewing radiograph, while the periapical radiograph revealed absence of pathology surrounding the remaining root, and adequate height of bone beyond the apex of the root to the floor of the sinus (Fig. 2).
Figure 1A

Figure 1B

Clinical preoperative photographs of the maxillary right posterior region showing fractured tooth #4 surrounded with heavily restored teeth.
Figure 2A

Figure 2B

Intraoral Periapical radiograph of tooth #4 and bitewing radiograph of the right posterior region.
Several treatment options were presented to the patient; however, the patient refused to involve the adjacent teeth #3 & #5 and preferred a treatment that would maintain these teeth in their current state. Therefore, both Crown Lengthening Surgery and FPD options were eliminated. The patient was originally referred for implant placement and favored this treatment approach.
A limited view CBCT (Fig. 3) was obtained which revealed several important findings:
• Absence of periapical pathology surrounding the remaining root.
• Plentiful height of bone between the apex of the remaining root and floor of the sinus.
• Mild dilaceration of the root, which needed to be taken into consideration during preparation of the osteotomy.
• Presence of a thick intact buccal plate at an appropriate height compared to the adjacent teeth.
• Location of the remaining root within the socket more toward the palatal aspect which resulted in thin palatal bone. This prevented the planned implant position to be anchored against the palatal bone as usually recommended for immediate implant placement.
• These findings were favorable for immediate implant placement and allowed for proper planning and selection of the appropriate diameter and length of the dental implant. Prior to surgery, the patient was informed of this treatment plan and she provided written informed consent.
Figure 3A
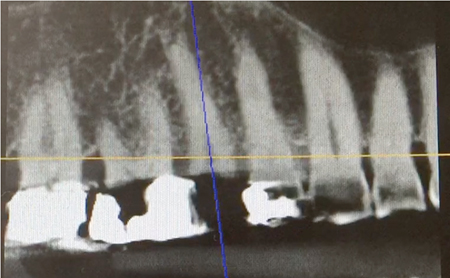
Figure 3B

Images A & B show panoramic and axial sections of the CBCT scan for tooth #4.
Case Management
After administration of local anesthesia via infiltrations on the buccal and palatal aspects of the upper right posterior quadrant, periotomes were used around the remaining root #4 and then extraction was performed with a rotating motion using root tip forceps while avoiding pressure on the socket walls. After extraction, thorough curettage of the socket and irrigation with saline was performed. No gingival flaps were raised and inspection of the socket revealed intact buccal and palatal bony walls (Fig. 4).
Figure 4

Clinical intraoperative photograph showing extraction of tooth #4 using root tip forceps.
Based on the literature, recommendations for immediate implant placement include maintaining a minimum 2.0mm buccal gap, which is grafted after implant placement to compensate for the bone remodeling which naturally occurs during the healing process.35,36 In addition, in this case due to the thin palatal bone, the implant was planned in the center of the socket allowing for formation of buccal and palatal gaps, which would be grafted. A Lindemann Burr was used with copious saline irrigation to create this new path for the implant osteotomy in the center of the socket and parallel to the adjacent roots avoiding the distal curve of the extraction socket, while maintaining these buccal and palatal gaps. Finally, the Astra EV drilling protocol was followed according to the manufacturer’s instructions for preparation of the osteotomy and placement of a 4.2X13mm implant (Fig. 5).
Figure 5A

Figure 5B

Clinical photograph of Lindemann Bur (A) and intraoperative photograph immediately after implant placement (B).
Primary stability was achieved at 45 NCM and initially a cover screw was placed in order to facilitate grafting the buccal and palatal gaps. A slow resorbing deproteinized bovine bone mineral (DBBM) was the utilized graft and subsequently the cover screw was replaced with a healing abutment. Resorbable Collatape material was placed surrounding the healing abutment and no sutures were needed as seen in Figure 6.
Figure 6A

Figure 6B

Figure 6C

Figure 6D

Clinical intraoperative photograph showing implant with cover screw (A), Immediately after grafting the buccal and palatal gaps (B), After replacing the cover screw with a healing abutment and placement of resorbable collatape material over the graft (C). Figure 6D shows periapical radiograph of implant #4.
The patient was instructed to follow a cold healthy liquid diet for the first 24 hours, followed by a soft diet for the remaining week and eat on the left side only. Patient was instructed to refrain from oral hygiene practices in the surgical site while rinsing with 0.12% chlorhexidine gluconate (two times daily) for two weeks, take 500 mg amoxicillin (every eight hours) for seven days and 400 mg ibuprofen (every eight hours) as needed for discomfort. At the one-week postoperative visit, the patient reported no discomfort or complications, oral hygiene was good and the gingival tissues appeared healing uneventfully. Buccal and palatal gaps surrounding the healing abutment were filled with fibrin-like tissue matrix as seen in Figure 7. The healing abutment and adjacent teeth were de-plaqued with a Q-tip soaked with Chlorhexidine.
Figure 7

Clinical photograph of the surgical site at the 1-week postoperative visit.
The patient was seen by the restoring dentist a few days after for delivery of an interim RPD. Fit Checker was used to make sure no pressure was on the implant and surrounding buccal and palatal aspects. The Fit Checker paste applied on the underlying surface of the RPD revealed no pressure on the implant, however, some pressure on the buccal aspect was evident by the extremely thin layer of paste on the flange (Fig. 8). This area was adjusted to prevent pressure on the buccal bone. The patient was instructed to avoid eating on the RPD and only to use it when needed for esthetic purposes.
Figure 8A

Figure 8B

Figure 8C

Clinical photographs showing the interim RPD (A), the Fit Checker Paste revealing the pressure area on the buccal aspect of the flange, and occlusal view of the interim RPD fully seated in place (C).
The patient returned at the two and six-week postoperative visits as seen in Figure 9 with uneventful healing and was seen three months later for a Periodontal Maintenance Prophylaxis and Final Implant Evaluation prior to referral back to the restoring dentist for restoration of the implant.
Figure 9A

Figure 9B

Clinical photographs of the surgical site at the two-week (A) and six-week (B) postoperative visits.
Figure 10A

Figure 10B

(A) Intraoral bitewing radiograph taken by the restoring dentist of the screw-retained provisional on implant #4. (B) Intraoral periapical radiograph taken by the restoring dentist of the final screw-retained implant crown.
Figure 11A

Figure 11B

(A) Clinical photographs taken at one-year postoperative visit during the patient’s Annual Periodontal Evaluation. (B) Bitewing and periapical radiographs of implant #4 at the one-year postoperative visit.
Clinical Outcomes
After three months of healing, the restoring dentist fabricated a screw-retained provisional for implant #4. The final implant crown was then delivered one month after by the restoring dentist (Fig. 10). The patient was on a three-month Periodontal Maintenance Recall and an Annual Periodontal Evaluation Recall. Clinical examination at the Annual Periodontal Evaluation revealed healthy gingival tissues surrounding the implant and radiographs were taken which revealed healthy and stable bone levels around implant #4 (Fig. 11). The patient was recommended by the restoring dentist over the past year to address the surrounding old restorations and open crown margins, however, due to financial reasons the patient will continue to be monitored closely for any development of caries or pathology and is aware of the risks involved. OH
Oral Health welcomes this original article.
References
- Pagni, G., Pellegrini, G., Giannobile, W. & Rasperini G. (2012) Postextraction Alveolar Ridge Preservation: Biological Basis and Treatments. International Journal of Dentistry, 2012, 151030.
- Rocchietta I., Fontana F., Simion M. Clinical outcomes of vertical bone augmentation to enable dental implant placement: A
systematic review. J. Clin. Periodontol. 2008;35:203–215. - Smith WR, Stahel PF, Suzuki T, Gabrielle P. Chapter 2. Musculoskeletal Trauma Surgery. In: Skinner HB, McMahon PJ. Eds. Current Diagnosis & Treatment in Orthopedics, 5e New York, NY: McGraw-Hill: 2014.
- Newman, Michael, Henry Takei, Perry Klokkevold, Fermin Carranza. Carranza’s Clinical Periodontology, 12th Edition. W.B. Saunders Company, 2015.
- Hupp, James, Myron Tucker, Edward Ellis. Contemporary Oral and Maxillofacial Surgery, 6th Edition. Mosby, 2014.
- Bauer TW, Muschler GF. Bone graft materials. An overview of the basic science. Clin Orthop Relat Res. 2000;371:10–27.
- Sadeghi R, Babaei M, Miremadi SA, Abbas FM. A randomized controlled evaluation of alveolar ridge preservation following tooth extraction using deproteinized bovine bone mineral and demineralized freeze-dried bone allograft. Dental Research Journal. 2016;13(2):151-159.
- Jambhekar S, Kernen F, Bidra AS. Clinical and histologic outcomes of socket grafting after flapless tooth extraction: a systematic review of randomized controlled clinical trials J Prosthet Dent. 2015 May; 113(5):371-82.
- Iasella JM., Greenwell H., Miller RL., Hill M., Drisko C., Bohra AA., Scheetz JP. Ridge preservation with freeze-dried bone allograft and collagen membrane compared to extraction site alone for implant site development: a clinical and histological study in humans. J Periodontol. 2003 Jul;74(7):990-999.
- Schwartz, Z, Mellonig, JT, Carnes, DL Jr., et al.: Ability of commercial demineralized freeze-dried bone allograft to induce new bone formation. J Periodontol. 67, 1996, 918–926.
- Schwartz, Z, Somers, A, Mellonig, JT, et al.: Ability of commercial demineralized freeze-dried bone allograft to induce new bone formation is dependent on donor age but not gender. J Periodontol. 69, 1998, 470–478.
- Murata M, Akazawa T, Mitsugi M, Kabir MA, UM IW, Minamida Y, et al. Autograft of dentin materials for bone regeneration. In: Pignatello R, editor. Advances in Biomaterials Sciences and Biomedical Applications. 1st ed. Croatia: InTech; 2013. pp. 391–404.
- Joshi CP, D’Lima CB, Samat UC, Karde PA, Patil AG, Dani NH. Comparative Alveolar Ridge Preservation Using Allogenous Tooth Graft versus Free-dried Bone Allograft: A Randomized, Controlled, Prospective, Clinical Pilot Study. Contemporary Clinical Dentistry. 2017;8(2):211-217.
- Sakkas, Andreas et al. “Autogenous Bone Grafts in Oral Implantology – is It Still a ‘gold Standard’? A Consecutive Review of 279 Patients with 456 Clinical Procedures.” International Journal of Implant Dentistry 3 (2017): 23. PMC. Web. 3 Sept. 2017.
- Moses O, Nemcovsky CE, Langer Y, Tal H. Severely resorbed mandible treated with iliac crest autogenous bone graft and dental implants: 17-year follow-up. Int J Oral Maxillofac Implants. 2007;22:1017–1021.
- Chiapasco M, Zaniboni M, Rimondini L. Autogenous onlay bone grafts vs. alveolar distraction osteogenesis for the correction of vertically deficient edentulous ridges: a 2–4-year prospective study on humans. Clin Oral Implants Res. 2007;18:432–440.
- Jensen SS, Terheyden H. Bone augmentation procedures in localized defects in the alveolar ridge: clinical results with different bone grafts and bone-substitute materials. Int J Oral Maxillofac Implants. 2009;24:218–236.
- Altiparmak N, Soydan SS, Uckan S. The effect of conventional surgery and piezoelectric surgery bone harvesting techniques on the donor site morbidity of the mandibular ramus and symphysis. Int J Oral Maxillofac Surg. 2015;44:1131–1137.
- Dahlin C, Linde A, Gottlow J, Nyman S. Healing of bone defects by guided tissue regeneration. Plast Reconstr Surg. 1988;81:672–676.
- Soldatos N, Stylianou P, Koidou V, Angelov N, Yukna R, Romanos G. Limitations and options using resorbable versus nonresorbable membranes for successful guided bone regeneration. Quintessence International [serial online]. February 2017;48(2):131-147.
- Sheikh, Zeeshan et al. “Natural Graft Tissues and Synthetic Biomaterials for Periodontal and Alveolar Bone Reconstructive Applications: A Review.” Biomaterials Research 21 (2017): 9.
- Caffesse, RG, et al. “Clinical Comparison of Resorbable and Non-Resorbable Barriers for Guided Periodontal Tissue Regeneration.” Journal of Clinical Periodontology, vol. 24, no. 10, Oct. 1997, pp. 747-752.
- Smith MacDonald E, Nowzari H, Contreras A, Flynn J, Morrison J, Slots J. Clinical and microbiological evaluation of a bioabsorbable and a nonresorbable barrier membrane in the treatment of periodontal intraosseous lesions. Journal Of Periodontology [serial online]. April 1998;69(4):445-453.
- Arbab H, Greenwell H, Allan N, et al. Ridge Preservation Comparing a Nonresorbable PTFE Membrane to a Resorbable Collagen Membrane: a Clinical and Histologic Study in Humans. Implant Dentistry [serial online]. 2016;2016(1):128-134.
- Iocca O, Farcomeni A, Pardiñas Lopez S, Talib H. Alveolar ridge preservation after tooth extraction: a Bayesian Network
meta-analysis of grafting materials efficacy on prevention of bone height and width reduction. Journal Of Clinical Periodontology [serial online]. January 2017;44(1):104-114. - Avila-Ortiz G, Elangovan S, Kramer K, Blanchette D, Dawson D. Effect of alveolar ridge preservation after tooth extraction: a systematic review and meta-analysis. Journal Of Dental Research [serial online]. October 2014;93(10):950-958.
- Ridge Preservation and Bone Grafting for the General Dental Practitioner. Jon B Suzuki, Diana Bronstein, Oral Health Group, 12-01-13
- Collagen Plug Application in Extraction Sockets. Suzuki JB,
Bronstein D., Implant Practice US. 2013;6(6):14-20. - Guided Bone Regeneration for Mandibular Impants. Jon B Suzuki, Diana Bronstein, Journal of the Massachusetts Dental Society 2013;62(1):12-15.
- Ridge preservation and advanced bone grafting for the general practitioner. Jon B Suzuki, Diana Bronstein, Journal of the Massachusetts Dental Society 01/2011; 60(3):40-2.
- Contemporary Esthetic Periodontics. Bronstein D, Suzuki K,
Garashi M, Suzuki JB. StomaEduJ. 2016;3(2):26-36. - Bronstein, D., & Garashi, M. Implant Placement With Augmentation and Osteotomy Sinus Elevation.
- Bronstein, D., Garashi, M., & Kravchenko, D. (2017). Extraction and Ridge Preservation Followed by Delayed Implant Placement of a Second Mandibular Molar with Proxim-ity to the Inferior Alveolar Nerve. Int J Dentistry Oral Sci, 4(3), 434-438.
- Misch, C. E. (2007). Contemporary Implant Dentistry-E-Book. Elsevier Health Sciences.
- Chen ST, Darby IB, Reynolds EC. A prospective clinical study of non-submerged immediate implants: clinical outcomes and esthetic results. Clinical Oral Implants Research. 2007 Oct 1;18(5):552-62
- Buser D, Martin W, Belser UC. Optimizing esthetics for implant restorations in the anterior maxilla: anatomic and surgical considerations. International Journal of Oral & Maxillofacial Implants. 2004 Nov 2;19(7).
 Mehdi Garashi, DDS, is a US-trained dentist and periodontist pursuing an MS Degree at Nova Southeastern University College of Dental Medicine in Fort Lauderdale, Florida, USA.
Mehdi Garashi, DDS, is a US-trained dentist and periodontist pursuing an MS Degree at Nova Southeastern University College of Dental Medicine in Fort Lauderdale, Florida, USA.
 Dr. Bronstein is an Associate Professor and the Associate Predoctoral Program Director in the Periodontology Department at Nova Southeastern University (NSU) College of Dental Medicine. She is a licensed dentist in the United States and Europe and is double boarded as a Diplomate of the American Board of Periodontology and a Diplomate and Fellow of the International Congress of Oral Implantology. She is a Periodontist and her Specialty Certification in Periodontology and Oral Implantology is from Temple University Kornberg School of Dentistry, as well as a Master of Science Degree in Oral Biology and a Master of Science in Medical Education from NSU. Dr. Bronstein has a Diploma in Clinical Homeopathy and has an extensive presentation and authoring background.
Dr. Bronstein is an Associate Professor and the Associate Predoctoral Program Director in the Periodontology Department at Nova Southeastern University (NSU) College of Dental Medicine. She is a licensed dentist in the United States and Europe and is double boarded as a Diplomate of the American Board of Periodontology and a Diplomate and Fellow of the International Congress of Oral Implantology. She is a Periodontist and her Specialty Certification in Periodontology and Oral Implantology is from Temple University Kornberg School of Dentistry, as well as a Master of Science Degree in Oral Biology and a Master of Science in Medical Education from NSU. Dr. Bronstein has a Diploma in Clinical Homeopathy and has an extensive presentation and authoring background.
 Denae C. Rushing, Doctor of Dental Medicine Candidate C/O 2019, Nova Southeastern University College of Dental Medicine, Plantation, FL.
Denae C. Rushing, Doctor of Dental Medicine Candidate C/O 2019, Nova Southeastern University College of Dental Medicine, Plantation, FL.
 Dr. Jon Suzuki is Professor of Microbiology and Immunology (School of Medicine) and Professor of Periodontology and Oral Implantology (School of Dentistry) at Temple University, Philadelphia, PA. USA. He served as Chairman and Director of Graduate Periodontology and Oral Implantology, and Associate Dean for Graduate Education at Temple University. He also served as Dean, Chief of Hospital Dentistry, and CEO of the Faculty Practice Plan at the University of Pittsburgh, USA.
Dr. Jon Suzuki is Professor of Microbiology and Immunology (School of Medicine) and Professor of Periodontology and Oral Implantology (School of Dentistry) at Temple University, Philadelphia, PA. USA. He served as Chairman and Director of Graduate Periodontology and Oral Implantology, and Associate Dean for Graduate Education at Temple University. He also served as Dean, Chief of Hospital Dentistry, and CEO of the Faculty Practice Plan at the University of Pittsburgh, USA.
RELATED ARTICLE: Improved Alveolar Ridge Augmentation Using Synthetic Monetite Based Onlay Grafts









