Introduction
Dental implant placement and implant-supported rehabilitations are highly successful treatment options, and more implants are being placed recently by general dental practitioners and specialists than ever before.1 For dental implant treatment to be successful, it is imperative to have a firm and stable osseointegration. Where, osseointegration is defined as the direct structural and functional connection at the interface between bone tissue and the dental implant surface.2 Therefore, bone formation, remodeling and metabolism plays a crucial role in the success of osseointegration.2,3 Aberrant bone metabolism has been shown to have a negative impact on osseointegration which can result in implant failure.3 Implant failures can be classified as either early failure, which occur before the prosthesis is placed, and late failures, which are associated with functional loading following placement of the prosthesis.4 Early failures frequently occur because of a disruption during the initial healing phase post-implantation, leading to impaired bone-to-implant contact and the subsequent failure of osseointegration;5 the onset of late failures may be related to multiple variables such as peri-implantitis,6 systemic factors,7,8 overloading,9 and/or parafunctional habits.7,9,10
Bone metabolism is thought to be affected by several factors thus interfering with the quality of osseointegration.3 The prevalence of systemic diseases and the related intake of medications has increased as the population ages. The intake of medications prescribed for some systemic diseases and conditions could potentially modulate bone metabolism and negatively influence implant-related outcomes with an increased risk of breakdown of the peri-implant tissues.11 Here we briefly discuss two such medications which are the Proton pump inhibitors (PPIs) and Selective serotonin reuptake inhibitors (SSRIs), and bring to the notice of our peers their potential association with dental implant failures.
Proton pump inhibitors (PPIs) are a group of drugs that are rapidly becoming the third most prescribed pharmaceutical products worldwide.12 PPIs are very effective in both prevention and treatment of gastrointestinal acid related conditions, such as peptic ulceration, gastroesophageal reflux disease (GERD or GORD), dyspepsia, helicobacter pylori infections, stress gastritis and eosinophilic esophagitis.13 PPIs irreversibly inhibit the proton pump in the acid-secreting parietal cells of the stomach and thereby suppress the gastric acidity.14 PPIs supress gastric acidity by inhibiting the functions of the proton pump (H1/K1 ATPase),14 which can also be found in bone tissue.15 The proton pump inhibition of the osteoclasts can decrease their activities and PPIs also impair calcium uptake through the intestines.16
Several observational studies have shown an association between the use of PPIs and high risk of bone loss and bone fractures.17 Also, animal studies have shown that PPIs administration in vivo can impair bone healing and implant osseointegration 18. Within the evidence available, systematic reviews have showed an association of PPIs with an increased dental implant failure.19 There is an increase in patients that have had successfully osseointegrated dental implant that have been in function and are failing after they are prescribed PPIs (Fig. 1). Despite the known negative effects of PPIs on the skeleton, the effect of these drugs has yet not been thoroughly explored in many important bone-related clinical conditions including osseointegrated dental implants. Many patients undergoing implant therapy are already taking PPIs without much thought given to their potential effect on osseointegrated/osseointegrating dental implants.
Fig. 1
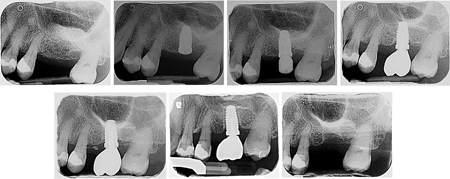
at #2.6 site (March 2017). 1C. Implant stage II procedure performed (June 2017). 1D. Implant #2.6 restored (November 2017).
1E. Follow-up appointment and continued maintenance therapy with no issues (November 2018). In 2019, the patient had a flare up of Irritable-Bowel-Syndrome (IBS) and was prescribed Pentaprazole (PPI) and Pinaverium Bromide. 1F. The implant was loose and there was loss of osseointegration (March 2021). 1G. The implant was lost as it fell out (April 2022)
Selective serotonin reuptake inhibitors (SSRIs) are prescribed for the treatment and/or management of depressive, or anxiety conditions. We are seeing a major increase in patients taking these medications. There is evidence which points towards an association of SSRIs with increased dental implant failure rate19. We see in our clinical practice more and more patients who have been placed on SSRI therapy after the implants have been loaded a few years back and are now demonstrating peri-implant bone loss (Fig. 2). Recent evidence suggests that SSRIs have been identified in playing a pivotal role on the osteoblast/osteoclast balance. Serotonin can regulate osteoclast activation and differentiation as osteoclasts derive from hematopoietic cell precursors.20 Furthermore, SSRIs have been shown to produce a detrimental effect on bone mineral density and trabecular microarchitecture through their anti-anabolic skeletal effects.21 Noteworthy, adding to the effect on osteoclast activation, SSRIs may increase osteoclast differentiation and reduce osteogenic differentiation and mineralization, which may also negatively impact implant osseointegration. Recently, a preclinical in vivo study has elucidated the effect of SSRIs on osteoblast differentiation and bone regeneration in rats.22 SSRI medication significantly reduced osteogenic differentiation and mineralization with concomitant reduction of osteoblast marker genes (including alkaline phosphatase, Osterix, and osteocalcin), indicating its putative impact on the regulation of bone metabolism.22 Such cellular findings are in concordance with the results obtained by Wu et al. (2014), who demonstrated that patients taking SSRIs experienced an increased risk of dental implant failure.23
Fig. 2

Recent evidence and findings from systematic reviews show an association of PPIs and SSRIs with increased implant failure.19 The effect of these medications requires further investigation in future studies as potential confounders for implant outcomes. A comprehensive evaluation and understanding of the patient’s medical background and the medication-specific side effects on bone metabolism, is necessary in assessing the patient’s risk of implant complications when considering dental implant therapy. We suggest discussing this with the patient during the treatment planning stage and being mindful of these implants having a reduced success rate.
Oral Health welcomes this original article.
References
- Gaviria, L., et al., Current trends in dental implants. 2014. 40(2): p. 50.
- Jokstad, A., Osseointegration and dental implants. 2009: John Wiley & Sons.
- Insua, A., et al., Basis of bone metabolism around dental implants during osseointegration and peri‐implant bone loss. 2017. 105(7): p. 2075-2089.
- Moy, P.K., et al., Dental implant failure rates and associated risk factors. 2005. 20(4).
- Baqain, Z.H., et al., Early dental implant failure: risk factors. 2012. 50(3): p. 239-243.
- Prathapachandran, J. and N.J.D.r.j. Suresh, Management of peri-implantitis. 2012. 9(5): p. 516.
- Do, T.A., et al., Risk factors related to late failure of dental implant—A systematic review of recent studies. 2020. 17(11): p. 3931.
- Paquette, D.W., N. Brodala, and R.C.J.D.C. Williams, Risk factors for endosseous dental implant failure. 2006. 50(3): p. 361-374.
- Sadowsky, S.J.J.I.j.o.i.d., Occlusal overload with dental implants: a review. 2019. 5(1): p. 1-5.
- Chatzopoulos, G.S. and L.F.J.C. Wolff, Symptoms of temporomandibular disorder, self-reported bruxism, and the risk of implant failure: A retrospective analysis. 2018.
- Aghaloo, T., et al., The Effects of Systemic Diseases and Medications on Implant Osseointegration: A Systematic Review. 2019. 34.
- Mazer-Amirshahi, M., et al., Rising rates of proton pump inhibitor prescribing in US emergency departments. 2014. 32(6): p. 618-622.
- Metz, D.C.J.D., Potential uses of intravenous proton pump inhibitors to control gastric acid secretion. 2000. 62(2-3): p. 73-81.
- Mullin, J.M., et al., Proton pump inhibitors: actions and reactions. 2009. 14(13-14): p. 647-660.
- Liu, J., et al., Proton pump inhibitors therapy and risk of bone diseases: An update meta-analysis. 2019. 218: p. 213-223.
- Wright, M.J., et al., Proton pump-inhibiting drugs, calcium homeostasis, and bone health. 2008. 66(2): p. 103-108.
- Abrahamsen, B. and P.J.B. Vestergaard, Proton pump inhibitor use and fracture risk—effect modification by histamine H1 receptor blockade. Observational case–control study using National Prescription Data. 2013. 57(1): p. 269-271.
- Al Subaie, A., et al., Systemic administration of omeprazole interferes with bone healing and implant osseointegration: an in vivo study on rat tibiae. 2016. 43(2): p. 193-203.
- Chappuis, V., et al., Medication-related dental implant failure: systematic review and meta-analysis. 2018. 29: p. 55-68.
- Battaglino, R., et al., Serotonin regulates osteoclast differentiation through its transporter. 2004. 19(9): p. 1420-1431.
- Kahl, K.G., et al., Bone mineral density, bone turnover, and osteoprotegerin in depressed women with and without borderline personality disorder. 2006. 68(5): p. 669-674.
- Nam, S.S., et al., Serotonin inhibits osteoblast differentiation and bone regeneration in rats. 2016. 87(4): p. 461-469.
- Wu, X., et al., Proton pump inhibitors and the risk of osseointegrated dental implant failure: a cohort study. 2017. 19(2): p. 222-232.
About the Author
 Dr. Zeeshan Sheikh is a Fellow of the Royal College of Dentist of Canada and Clinical Scientist in Periodontics and Assistant Professor at Dalhousie University (departments of Applied Oral Sciences, Dental Clinical Sciences & Biomedical Engineering).
Dr. Zeeshan Sheikh is a Fellow of the Royal College of Dentist of Canada and Clinical Scientist in Periodontics and Assistant Professor at Dalhousie University (departments of Applied Oral Sciences, Dental Clinical Sciences & Biomedical Engineering).
 Dr. Aditya Patel is a Fellow of the Royal College of Dentists of Canada and the current President of the Canadian Academy of Periodontology. He works in private practice in Nova Scotia.
Dr. Aditya Patel is a Fellow of the Royal College of Dentists of Canada and the current President of the Canadian Academy of Periodontology. He works in private practice in Nova Scotia.
 Dr. Eraldo L. Batista Jr. is a Fellow of the Royal College of Dentist of Canada and Associate Professor and Head of the Division of Periodontics at Dalhousie University, and Periodontist at the IWK Health Centre.
Dr. Eraldo L. Batista Jr. is a Fellow of the Royal College of Dentist of Canada and Associate Professor and Head of the Division of Periodontics at Dalhousie University, and Periodontist at the IWK Health Centre.













