NOTE: GRAPHIC IMAGES
Introduction
Necrotizing soft tissue infections of the head and neck constitute a small proportion of all head and neck infections, but have significant morbidity and historically mortality rates of up to 50%. 1-4 Necrotizing infections may be of odontogenic or non-odontogenic origin. Common non-odontogenic sources of head and neck infections in healthy patients include peritonsillar/tonsillar, otologic (otitis media, otitis externa), sino-nasal and in situations of trauma. Dental professionals must be aware of the signs and symptoms of necrotizing soft tissue infections in order to appropriately initiate hospital based medical and surgical treatment. This article will present two unusual cases of necrotizing infection of the head and neck and will review the presentation, diagnosis and management of these infections.
Case 1: Odontogenic Scalp Infection
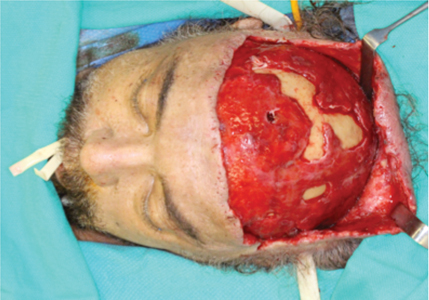
A 61-year-old male with history of hypertension, poorly controlled type 2 diabetes mellitus, and >25 pack year history of smoking was transferred to our tertiary care hospital centre after initial attempts to manage an aggressive infection of the scalp at a peripheral hospital. This patient endorsed a four-week history of painful right midfacial swelling at the time of presentation to the peripheral emergency department. Prior to transfer to our centre, the patient underwent two incision and drainage procedures six days apart utilizing a transverse frontal scalp/forehead incision as well as a right maxillary vestibular incision. Radiographic evidence of osteolysis was present in the region of carious teeth 16 and 17; these teeth were extracted at the time of the first incision and drainage at the peripheral hospital as they were the only identifiable source of infection. At the peripheral hospital, the patient was managed in the intensive care unit for hemodynamic and electrolyte abnormalities which were stabilized prior to transfer to our centre. At the time of transfer, a contrast enhanced computed tomography (CT) of the head and neck was obtained, which revealed an extensive, partially drained abscess involving the bilateral frontal, temporal and parietal scalp as well as multiloculated abscess within the right temporalis muscle and masticator space (Fig. 1). The patient was taken to the operating room for incision and drainage of the aforementioned spaces. Extensive necrosis of the galea aponeurotica, pericranium and subcutaneous tissue of the scalp was appreciated (Fig. 2); this tissue was aggressively debrided to vital bone/tissue and multiple drains were placed. Due to the aggressive nature of this infection, two additional operating room visits were required at our tertiary care hospital for further debridement of the infected tissue. Due to the unfortunate placement of the initial incision, access to the occipital scalp was not possible through the frontal scalp incision, and a sagittal occipital scalp incision was required to access the posterior and inferior aspect of this infection near the superior posterior neck. Throughout this course in hospital, the infectious disease team and intensive care teams were involved in patient care. Cultures from these operations revealed growth of P. Miribalis and S. Constellatus, which were susceptible to moxifloxacin. After 14 days in peripheral hospital and 24 days in our hospital, five operating room visits and numerous hours of wound care, this patient was discharged on a one-month outpatient course of Moxifloxacin. Outpatient follow up revealed resolution of the infection and at post-discharge week two, the sagittal occipital incision was closed in a tertiary fashion.
Fig. 1
Select contrast enhanced CT images demonstrating multiloculated abscess collections with subcutaneous air.


Fig. 2
Intraoperative photo demonstrating variable areas of necrosis of the pericranium, galea aponeurotica and subcutaneous connective tissue. The unfavourable incision to access to the vertex of the skull was performed at an outside hospital centre prior to transferring the patient to our care.
Case 2: Non-Odontogenic Lip Infection
A 25-year-old male with unremarkable past medical history presented with four days of progressively worsening lower lip and chin swelling. The initial assessment was remarkable for tachycardia and a cellulitic process of the lower lip and chin without evidence of fluctuance, crepitus or tissue necrosis. Low grade left sided chest pain was present on inspiration. Initial lab findings were remarkable for leukocytosis with marked bandemia, hyponatremia and mild transaminitis.
Diagnostic imaging included contrast enhanced CT of the face, which revealed evidence of edema of the lower lip and chin without gas formation or fluid collection as well as a non-occlusive thrombus of the left common facial vein. A diagnosis of lower lip and chin cellulitis with thrombophlebitis of the left common facial vein was made (Fig. 3). The patient was admitted and antimicrobial therapy with intravenous (IV) ampicillin-subactam and vancomycin was initiated. On post admission day one, the patient developed worsening tachycardia (heart rate >150) and required supplemental oxygen. The clinical exam was notable for a worsening left sided lower lip and chin fullness with concern for development of an abscess. Additionally, significant bilateral chest pain was present on deep inspiration. A CT angiogram of the chest was obtained which revealed numerous lung nodules and small bilateral pleural effusions (Fig. 4). Internal Medicine was consulted; we initiated a therapeutic heparin infusion and bedside incision and drainage of the lower lip and chin was performed under local anaesthesia. At this time, the plan included a trial of medical management with plan to pursue further surgical care if the patient did not improve over the following 12-24 hours. On post admission day three, the patient remained septic with worsening fever. He was subsequently taken to the operating room for ligation and resection of the left common facial vein and thrombus and repeat incision and drainage with debridement of the lower lip and chin (Figs. 5-7). The intraoperative course was uneventful. While in the post-anaesthesia care unit, it was noted that the patient developed worsening tachycardia, tachypnea, hypotension, and interval drastic worsening lower lip and chin edema with an area of necrosis measuring 1 cm. 2 The patient was immediately taken back to the operating room for emergent intubation, resuscitation and further debridement of the lower lip necrosis. Post-operatively the patient remained intubated and was transferred to the intensive care unit.
At this time the patient’s problem list included a necrotizing soft tissue infection of the lower lip and chin with septic thrombophlebitis of left common facial vein, hypoxic respiratory failure, hyponatremia, transaminitis and septic shock. On post admission day four, no further tissue necrosis was identified, the patient was weaned from hemodynamic support and was extubated. During this process, the infectious disease service was consulted and antimicrobial therapy was broadened to piperacillin-tazobactom and vancomycin. After culture and sensitivity analysis was completed, antimicrobial therapy was changed to nafcillin, clindamycin and metronidazole. On post admission day five, he continued to have fevers and tachypnea ranging from 30 to 40 respirations/minute. Subsequent CT neck and chest revealed interval increase in pleural fluid accumulation prompting chest tube placement. During the following days, the patient’s respiratory status improved, his sepsis resolved, and chest tubes were removed. His definitive culture from both lower lip and facial vein grew methicillin sensitive Staphylococcus aureus; he was discharged on post admission day 20 with one-month of intravenous nafcillin.
Fig. 3
Coronal section of CT neck revealing presence of non-occlusive thrombus (arrow) of the left common facial vein.

Fig. 4
Axial image of CT chest obtained on hospital day 1 revealing bilateral pulmonary nodules and small bilateral effusions.

Fig. 5
Intra-operative photo demonstrating preoperative appearance of lower lip.

Fig. 6
Intraoperative photo revealing limited left neck dissection specimen including left common facial vein.

Fig. 7
Intraoperative photo revealing left neck defect from thrombectomy

Discussion
These case presentations demonstrate two unusual examples of necrotizing head and neck soft tissue infections. While healthy individuals can be affected, most commonly individuals with comorbidities such as obesity, immunosuppression, diabetes, renal failure, and individuals with history of substance abuse including longstanding smoking history are affected. 5 Due to the low incidence of necrotizing soft tissue infections of the head and neck combined with nonspecific initial findings, misdiagnosis is possible. Wang et al 6 have outlined a progressive staging of necrotizing soft tissue infections based on the progression of disease. Stage 1 involves tenderness, erythema, swelling and warmth of the involved tissue. Stage 2 involves blister or bullae formation, while Stage 3 includes crepitation, anaesthesia and necrosis of the involved skin. CT scans are essential to compliment the initial head and neck clinical exam and may demonstrate abscess collections, fat stranding and subcutaneous air in the region of infection. Careful clinical examination is important to help the clinician determine if radiographic findings of subcutaneous air/gas are due to a mucosal breach or due to gas producing organisms. Magnetic resonance imaging (MRI) may provide superior diagnostic accuracy in identification of necrotizing soft tissue infections, but is infrequently utilized as the initial diagnostic imaging modality in hospital settings. 7 Surgical exploration will reveal necrosis of the subcutaneous connective tissue spreading through the fascial planes of the involved anatomic region. Necrotizing infections of the head and neck are potentially fatal due to the propensity for spread to the chest, abdomen, head and/or through vascular erosion. Significant morbidity is seen due to damage of skin, muscle and potentially bone in the involved area. The principles of management include administration of broad spectrum antibiotics followed by culture directed antibiotic, aggressive, often repeated surgical exploration and debridement and frequent re-evaluation. Intravenous immunoglobulin and hyperbaric oxygen therapy are adjunct therapies to potentially improve the outcomes of patients refractory to initial antibiotic and surgical management. 3 Medical management of patients with necrotizing soft tissue infections frequently involves intensive care based therapy to monitor and treat hemodynamic, electrolyte and pulmonary abnormalities. With accurate and prompt diagnosis, early surgical and antimicrobial treatment can minimize the risk of mortality and minimize the morbidity associated with necrotizing head and neck infections. OH
Oral Health welcomes this original article.
References
- Hakkarainen, T. W., Kopari, N. M., Pham, T. N., & Evans, H. L. (2014). Necrotizing soft tissue infections: review and current concepts in treatment, systems of care, and outcomes. Current problems in surgery, 51(8), 344-362.
- Al-Ali MA, Hefny AF, Idris KM, Abu-Zidan FM. Cervical Necrotizing Fasciitis: An Overlooked Diagnosis of a Fatal Disease. Acta Otolaryngol. 2018 Apr;138(4):411-414. doi: 10.1080/00016489.2017.1393841.
- Wolf H, Rusan Hon M, Lambertsen K, Ovesen T. Necrotizing Fasciitis of the Head and Neck. Head Neck. 2010 Dec;32(12):1592-6. doi: 10.1002/hed.21367.
- Cuddy K, Saadat N, Khatib B, Patel A. Necrotizing Lip Infection Causing Septic Thrombophlebitis of the Neck: A Rare Variant of Lemierre Syndrome. J Oral Maxillofac Surg. 2018 Jan;76(1):134-139. doi: 10.1016/j.joms.2017.05.030.
- Inan CH, Yener HM, Yilmaz M, Gözen ED, Erdur ZB, Oroğlu B, Olcay E, Memmedova N. Cervical Necrotizing Fasciitis of Odontogenic Origin and Hyperbaric Oxygen Therapy. J Craniofac Surg. 2017 Oct;28(7):e691-e692. doi: 10.1097/SCS.0000000000003842.
- Wang YS, Wong CH, Tay YK. Staging of Necrotizing Fasciitis Based on the Evolving Cutaneous Features. Int J Dermatol. 2007 Oct;46(10):1036-41.
- Kim KT, Kim YJ, Won Lee J, Kim YJ, Park SW, Lim MK, Suh CH. Can necrotizing infectious fasciitis be differentiated from nonnecrotizing infectious fasciitis with MR imaging? Radiology. 2011 Jun;259(3):816-24. doi: 10.1148/radiol.11101164
About the Author
 Assistant Professor; Director of Education and Maxillofacial Trauma; Graduate Program in Oral and Maxillofacial Surgery; University of Toronto; Staff Surgeon: Mt. Sinai Hospital, Princess Margaret Hospital karl.cuddy@utoronto.ca
Assistant Professor; Director of Education and Maxillofacial Trauma; Graduate Program in Oral and Maxillofacial Surgery; University of Toronto; Staff Surgeon: Mt. Sinai Hospital, Princess Margaret Hospital karl.cuddy@utoronto.ca
RELATED ARTICLE: Nutritional Strategies in the Management of Necrotizing Ulcerative Periodontitis
Follow the Oral Health Group on Facebook, Instagram, Twitter and LinkedIn for the latest updates on news, clinical articles, practice management and more!












