Abstract
Objective: The objective of this study was to assess the effects of at-home carbonation systems on the pH of water, apple juice, and orange juice as it relates to enamel dissolution at a critical pH.
Methods: A 2x3x8 fractional factorial design was employed to determine the effects of at-home carbonation machines on water, apple juice, and orange juice. In total, five commercially available machines (two of which had multiple settings) were examined. Carbonation was performed at two temperatures, room temperature (25oC) and refrigeration temperature (4oC), to determine temperature effects on final pH after carbonation. Two sample t-tests were used to determine significance between pre- and post-treatment pH values. A Tukey test was used to compare pH alterations from the eight different carbonation machines/settings at the two specified temperatures.
Results: At-home carbonation systems lowered the pH of water below the critical pH of enamel (hydroxyapatite) (p < 0.01). At-home carbonation systems had a negligible effect on the pH of orange juice (MD = 0.012, p < 0.01), and did not affect the pH of apple juice (p = 0.34). Temperature had a significant effect on pH changes during carbonation such that carbonating lower temperature beverages resulted in a greater change to overall pH (p < 0.01). The greatest pH change was observed using the Fizzpod system on T=4oC water (MD = 3.257, SD = 0.021), whereas the smallest pH change was observed using the Spärkel system (Low Setting) at T=25oC (MD = 2.603, SD = 0.004).
Conclusion: At-home carbonation systems significantly increase the acidity of both water and orange juice, but not apple juice. FIZZpod® carbonation systems produced the most pH change, while the lowest was produced by Spärkel® (low-setting). Consumers should be aware of the effects these machines have on their beverages, and the possible sequelae of consuming beverages with erosive potential.
Introduction
Carbonated beverages comprise a significant proportion of Canadian beverage intake among both teen and adult populations (Garriguet). The process of carbonation involves the dissolution of gaseous CO2 into water, giving it its desirable effervescent quality. The resulting effervescence has become a staple of many popular consumer beverages, including most soft drinks. With the recent popularity of at-home carbonation machines, many individuals are electing to carbonate water and beverages at home, often with the assumption that doing so is less harmful than branded beverages. Of particular interest to dental practice is the resultant acidogenicity of the beverages due to the carbonation process. This study examines the resultant pH values of beverages treated with various popular consumer at-home carbonation machines and reviews the relevant literature in the context of factors affecting enamel dissolution.
Carbonation and the Generation of Acid
Carbonation is the process of dissolving gaseous CO2 in water, consequently resulting in some acid production before reaching equilibrium. This may be achieved either by injection of high-pressure CO2(g) or via chemical processes. The majority of at-home carbonation units such as those manufactured by Sodastream®, FIZZPod®, Drinkmate®, and AARKE® employ the high-pressure injection system. Other units, such as those made by Spärkel®, employ the use of a chemical reaction between granulated sodium bicarbonate and citric acid to release CO2(g). (Fig. 1) Upon release of CO2(g) into the aqueous solution, a reaction occurs with water to create carbonic acid (H2CO3). Carbonic acid is a major constituent contributing to the acidity of soda or carbonated drinks. Direct statements of acidity (e.g. the log acid dissociation constant (pKa)) for carbonic acid are difficult, as CO2 exists at equilibrium with H2CO3 in aqueous solution and the degree of carbonation may vary. However, it is understood that carbonic acid possesses considerable acid strength and contributes to the acidity of the aqueous solution in which it resides (Pines et. al).
Fig. 1

Fig. 2
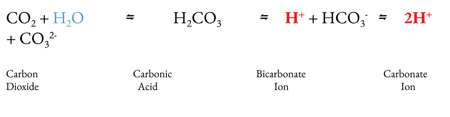
Fig. 3
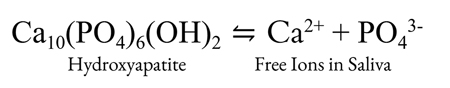
A Brief Review of Enamel Dissolution
Enamel is primarily composed of hydroxyapatite (HA). The chemical formula for HA is Ca10(PO4)6(OH)2. HA is in equilibrium with its constituent ions in an aqueous solution.
Changes in the concentrations of HA’s constituent ions, therefore, have an effect on where the equilibrium lies, in accordance with Le Chatalier’s Principle. An acidic solution would diminish the amount of free hydroxide (OH-) ions via the equation: H+ + OH- ↔ H2O. Similarly, the acidity of a solution determines which form inorganic phosphate takes in solution in accordance with the equilibrium equation in Figure 4.
Fig. 4

An increase in acidity shifts the equilibrium of inorganic phosphate such that the concentration of PO43- is reduced. The acidity dependent decrease in concentrations of free hydroxide and PO43- ions results in a shift to the right side of the HA equilibrium, therefore favoring dissolved HA. Although not affected by acidity, concentrations of calcium ions also contribute to this equilibrium.
Given that the HA of an individual tooth may contain certain impurities such as carbonate and fluoride, its solubility is somewhat variable. Similarly, the concentrations of ions found in oral plaque microenvironments may also vary and affect the solubility of HA. Despite these individual variabilities, the critical pH at which enamel (HA) tends to dissolve is generally cited around pH 5.5 (Dawes).
There is little evidence observing the quantitative effects of popular at-home carbonation machines on beverage pH. One study using Sodastream® products created water with a pH of 3.58 – 3.74 using manufacturer’s instructions (Ryu). Extrapolating effects from commercially available carbonated water is difficult due to a wide range of observed pH depending on the product (pH 3.58 – 5.87) and the comparative various levels of carbonation that may be provided with an at-home machine (Ryu, Lee, Kim). Thus, this study aimed to target the gap in knowledge by performing tests on at-home carbonation tools.
Methods
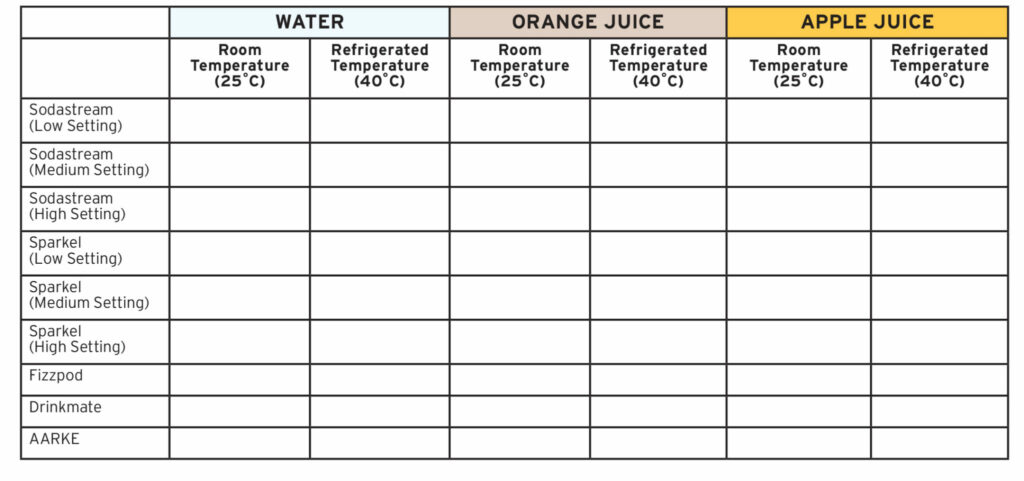
A 2x3x8 fractional factorial design was employed to determine the effects of at-home carbonation machines on water, apple juice, and orange juice, according to the matrix in Table 1. In total, five commercially available machines (two of which had multiple settings) were examined. The systems used represented commonly purchased products available in the Canadian market, and included: SodaStream® (SRA001, SN T8, Lot 11, 09/06/17), DrinkMate® CounterTop (06268, 06257, 06262, 07/30/19), Spärkel® Beverage System (20-07-0128), FIZZpod® Soda Maker (20200400034821), and AARKE® Carbonation System (20-056-6701).
Table 1
Bottles of water, orange juice, and apple juice were filled to the maximum amount, as per manufacturer instructions. Samples from each system were carbonated as per manufacturer instructions. Each system required use of bottles specific for that system, which had varying volumes. The varying volumes between systems were not considered a confounding variable as the aim of the experiment was to determine pH changes in situ for consumers. The water used for testing all samples was bottled Western Family® 500mL water bottles. SunRype® Apple Juice (from concentrate) and SunRype® Orange Juice (from concentrate) were used to test apple juice and orange juice carbonation. In total, 144 samples were taken, using triplicate sampling for each machine (and setting, where applicable) at each of the two predefined temperatures, according to the matrix defined in Table 1.
Carbonation was performed at two temperatures, room temperature (25oC) and refrigeration temperature (4oC), to determine temperature effects on pH. The pH of all samples was performed with the calibrated pH meter Preciva® PH320001. Temperature was analysed before and after testing using a digital thermometer.
Analyses were conducted using STATA14 (StataCorp, College Station, Texas). Replicates were averaged and their standard deviations were determined. Two sample t-test was used to determine significance between pre- and post-treatment pH values. A Tukey test was used to compare pH alterations from the eight different carbonation machines/settings at the two specified temperatures. Significance (alpha) was set at 0.05.
Results
pH measurements and temperature readings were taken prior to and after carbonation, with data recorded in Figure 5 and Table 1. Pre-treatment pH values were lower (more acidic) at T=25oC than at T=4oC (p < 0.01) for all solutions.
Fig. 5

Fig. 6

Fig. 7

Temperature had a significant effect on pH changes during carbonation. Post-treatment room temperature water was shown to be significantly less acidic than post-treatment cold water (r = 0.275, p < 0.01). Post-treatment room temperature orange juice was shown to be significantly more acidic than its post-treatment cold counterpart (r = 0.024, p < 0.01). Post-treatment room temperature apple juice room, however, was shown to have no significant difference from its post-treatment cold counterpart (p = 0.19).
Carbonation resulted in changes to the pH of water (MD = -2.93, p < 0.01) and orange juice (MD = .012, p < 0.01), but not apple juice (p = 0.34). While all carbonation machines produced significant changes in water pH, only Fizzpod and Sparkel (Low Setting) differed in the pH change produced when pooling data across both temperatures (MD = 358, p < 0.01). When controlling for temperature, Tukey tests run on separate T=4oC and T=25oC data sets found that 55 combinations of machines produced significantly different changes in pH from each other. (Table 2) When controlling for temperature, Fizzpod and Sparkel (Low Setting) had the greatest difference in carbonation pH change at both T=4oC (MD=0.37, p < 0.01) and at T=25oC (MD=0.347, p < 0.01). Carbonated water produced by Fizzpod had the highest pH change at T=4oC (MD= 3.257, SD = 0.021) and T=25oC (MD= 2.950, SD = 0.024). Carbonated water produced by Spärkel (Low Setting) had the lowest pH change at T=4oC (MD = 2.887, SD = 0.004) and T=25oC (MD = 2.603, SD = 0.004).
Table 2
Discussion
The purpose of the experiment was to determine the pH change of popular at-home carbonation machines on the pH of water, apple juice, and orange juice as they relate to tooth demineralization. We hypothesized that, under all conditions, carbonation would reduce the pH of the liquid in question, with greater effects seen at cold temperatures owing to increasing dissolved gas content as temperature decreases.
Expectations of temperature’s effects on carbonation were confirmed with pre- and post- measurement data. Literature had suggested that pH values of water would be lower (more acidic) at higher temperatures; our results found pre-treatment warm water pH values to be more acidic than pre-treatment cold water. This can be explained by water’s dissociation into constituent ions being an endothermic reaction. Thus, the addition of heat shifts the reactions Kw and thus results in a lower pH reading (Chem website). In the post-treatment measurements of
water, we observed a reversal in this trend with warm water having a higher pH. This trend is due to a decreased ability of carbon dioxide to remain soluble at higher temperatures and thus decreasing the available amount of carbonic acid product (Colt). It is important to note that the effect of temperature on erosive potential of beverages goes beyond pH alone. Enamel dissolution has been shown to be a diffusion-controlled reaction, and thus has a higher dissolution rate at high temperatures (Gray). Both enamel dissolution and erosion depth have been shown to increase with higher beverage temperatures (West, Eisenburger).
We also noted that post-treatment warm orange juice pH decreased significantly, but this was not seen in the apple juice group. The difference, however, between warm and cold post-treatment orange juice was small (r=0.024). Both apple and orange juice contain a wealth of organic acids, such as citric acid in orange juice and malic acid in apples. Though these constituents contribute to the drink’s acidity, they also contribute to their significant buffer capacities (Touyz, Edwards). In fact, fruit juices have been found to have the highest buffering capacity amongst a variety of commercially available beverages (Edwards, Singh). This buffering capacity is essential to maintaining a stable pH in the face of acid or base challenge. When looking at the effects of carbonation in the juice treatments, we observed no significant difference in apple juice. Orange juice post-treatment groups were found to be significantly more acidic, but once again this difference was small (MD =0.012). In a study by Singh et. al, apple juice was found to have a higher buffering capacity than orange juice. We hypothesize that apple juice’s higher buffering capacity may explain the discrepancy between the apple and orange juice groups. One may conclude from our trial that at-home carbonation significantly affects the pH of orange juice but not apple juice, and that the expected change in pH from carbonating orange juice is not clinically appreciable (pH MD = 0.01, SD = 0.004).
When observing the effect of carbonation on water, we found that pH was changed significantly across all groups. As outlined in Figure 5, one may observe that all of the at-home carbonation systems produced water with pH values well below the pH required for hydroxyapatite dissolution. Plain carbonated water has been shown to cause enamel dissolution in various studies (Ryu, Kim, Parry). This is further corroborated by the fact that degassing of sparkling mineral water reduced enamel dissolution (Parry). Of all the machines, the FIZZpod® (MD = 3.10, SD = 0.17) produced the greatest pH change, while Spärkel® (low-setting) (MD = 2.75, SD = 0.16) produced the least. This finding is of clinical significance as consumers should be aware of the relative acidity produced by at-home carbonation systems as a whole. Compared with other commercially available carbonated beverages (Fig. 5), our results suggest at-home carbonation systems produce a slightly more acidic water than commercially available carbonated waters, however the testing methodology for the comparator data was not delineated.
Although pH of a beverage contributes to its erosive potential, there are other factors to consider. Literature has suggested that the amount of titratable acid, thus taking into consideration its buffer capacity, is a more accurate representation of erosive potential (von Fraunhofer). This is due to the fact that it is more difficult for the oral environment to neutralize the pH of a liquid with a high buffering capacity. A study on beverages also found that initial pH values gave no indication as to its underlying buffer capacity (Edwards).
Additionally, mineral content of beverages may also affect the dissolution equilibrium. Addition or enrichment of beverages with calcium has been shown to decrease their erosive potential (Parry, Stefanski).
It is important to note that final water pH values presented in this study should not be taken as representative final pH for all treatments of these machines.
Final pH values will vary depending on the pH of the initially source water that the consumer may use.
Limitations
Only one machine from each company was tested, and each machine may not be an accurate representation of the model’s effects on pH change. Only one water source was used for testing; examining other standardized sources may have altered the final pH such that it may have fallen under the critical pH of fluorapatite dissolution.
Conclusion
At-home carbonation systems significantly increase the acidity of both water and orange juice, but not apple juice. However, the change in orange juice pH is not clinically appreciable. The lack of large change in pH values in the juices can be attributed to its buffer capacity, which is an important factor to consider when assessing the erosive potential of a beverage. FIZZpod® carbonation systems produced the most pH change, while the lowest was produced by Spärkel® (low-setting). Consumers should be aware of the effects these machines have on their beverages, and the possible sequelae of consuming beverages with erosive potential.
Oral Health welcomes this original article.
References
- Statista. (2018, February) Revenue of SodaStream worldwide from 2011 to 2017. https://www-statista-com.ezproxy.library.ubc.ca/statistics/653118/global-revenue-of-sodastream/
- Statista. (2019, July) Sales of the leading seltzer, bottled sparkling/mineral water brands of the United States in 2019. https://www-statista-com.ezproxy.library.ubc.ca/statistics/252416/sales-of-the-leading-bottled-sparkling-water-brands-in-the-us/
- Dawes C. What is the critical pH and why does a tooth dissolve in acid? J Can Dent Assoc. 2003 Dec;69(11):722-4. PubMed PMID: 14653937.
- Wang LJ, Tang R, Bonstein T, Bush P, Nancollas GH. Enamel demineralization in primary and permanent teeth. J Dent Res. 2006 Apr;85(4):359-63. PubMed PMID: 16567559; PubMed Central PMCID: PMC2691661.
- Ryu HK, Kim YD, Heo SS, Kim SC. Effect of carbonated water manufactured by a soda carbonator on etched or sealed enamel. Korean J Orthod. 2018 Jan;48(1):48-56. doi: 10.4041/kjod.2018.48.1.48. Epub 2017 Nov 19. PubMed PMID: 29291188; PubMed Central PMCID: PMC5702778.
- Lee HO. Effects of sparkling water on the surface of composite resin [dissertation] Gwangju: Chonnam National Uni; 2016.
- Kim SK, Park SW, Kang SM, Kwon HK, Kim BI. Assessment of the erosive potential of carbonated waters. J Korean Acad Oral Health. 2015;39:273–279.
- Reddy A, Norris DF, Momeni SS, Waldo B, Ruby JD. The pH of beverages in the United States. J Am Dent Assoc. 2016 Apr;147(4):255-63. doi: 10.1016/j.adaj.2015.10.019. Epub 2015 Dec 2. PubMed PMID: 26653863; PubMed Central PMCID: PMC4808596.
- Stefan´ ski, T., & Postek-Stefan´ ska, L. (2014). Possible ways of reducing dental erosive potential of acidic beverages. Australian dental journal, 59(3), 280-288.
- Wegehaupt, F. J., Günthart, N., Sener, B., & Attin, T. (2011). Prevention of erosive/abrasive enamel wear due to orange juice modified with dietary supplements. Oral diseases, 17(5), 508-514.
- Magalhães AC, Wiegand A, Rios D, Honório HM, Buzalaf MA. Insights into preventive measures for dental erosion. J Appl Oral Sci. 2009 Mar-Apr;17(2):75-86.
- Temperature Dependence of the pH of pure Water. (2020, August 15). Retrieved April 6, 2021, from https://chem.libretexts.org/@go/page/1293
- Dissolved Gas Concentration in Water Computation as Functions of Temperature, Salinity and Pressure by John Colt.
- TOUYZ, L.Z.G. (1994) The acidity (pH) and buffering capacity of Canadian fruit juice and dental implications. Journal of the Canadian Dental Association, 60, 454.
- Buffering capacities of soft drinks: the potential influence on dental erosion M. EDWARDS, S. L. CREANOR, R. H. FOYE & W. H. GILMOUR Hard Tissue Research Group, University of Glasgow Dental School, U.K.
- Singh S, Jindal R. Evaluating the buffering capacity of various soft drinks, fruit juices and tea. Journal of conservative dentistry: JCD. 2010 Jul;13(3):129.
- Gray 1962, Kinetics of the Dissolution of Human Dental Enamel in Acid. West 2000, Erosion of dentine and enamel in vitro by dietary acids: the effect of temperature, acid character, concentration and exposure time.
- Eisenburger 2003, Influence of liquid temperature and flow rate on enamel erosion and surface softening.
- Parry, 2001 Investigation of mineral waters and soft drinks in relation to dental erosion.
- Von Fraunhofer 2006, Dissolution of dental enamel in soft drinks.
About the Author
 Michael Siarkowski, is a graduate of the Faculty of Dentistry, The University of British Columbia, Vancouver, BC, Canada. He is currently in private practice in Calgary, Alberta.
Michael Siarkowski, is a graduate of the Faculty of Dentistry, The University of British Columbia, Vancouver, BC, Canada. He is currently in private practice in Calgary, Alberta.
 Antonio Atte, is a first year resident in Oral Maxillofacial Surgery program at the University of Oklahoma Health Sciences Center, Oklahoma City, Oklahoma, USA. Dr Atte is a graduate of the Faculty of Dentistry, The University of British Columbia, Vancouver, BC, Canada.
Antonio Atte, is a first year resident in Oral Maxillofacial Surgery program at the University of Oklahoma Health Sciences Center, Oklahoma City, Oklahoma, USA. Dr Atte is a graduate of the Faculty of Dentistry, The University of British Columbia, Vancouver, BC, Canada.
 Reza Nouri, is a Clinical Associate Professor, at the Faculty of Dentistry, The University of British Columbia, and is in private practice atPDG Pediatric Dental Group, Vancouver, BC, Canada.
Reza Nouri, is a Clinical Associate Professor, at the Faculty of Dentistry, The University of British Columbia, and is in private practice atPDG Pediatric Dental Group, Vancouver, BC, Canada.
Check out more articles from the 2022 Pediatric Dentistry issue!