Introduction
Metastatic dissemination to the jaws and oral cavity is a rare phenomenon for the natural course and progression of cancer. Amongst all oral malignancies, metastatic tumors account for only 1% of cases.1 However, in up to 25% of cases, a metastatic tumor in the oral cavity represents an occult malignancy or a primary tumor that has not yet been identified or diagnosed. Unfortunately, the diagnosis of a metastatic lesion to the jaws or oral cavity represents a late stage of disease with a poor prognosis.
It is important to distinguish that metastatic lesions can appear at either the oral soft tissues or the facial skeleton, since they differ in terms of their clinical and radiographic presentation, pathogenesis, as well as implications for the identity of the primary tumor. Metastatic tumors to the facial skeleton occur twice as frequently as those to the oral mucosa.2 Specifically within the facial skeleton, metastases to the mandible are more common than the maxilla, with the molar region being the most common (>50%), followed by the premolar region (38%), and the angle-ramus unit (29%).2-4 Amongst the oral mucosal sites, the attached gingiva is the most common site for metastases to appear (60%), followed by the tongue (18%).5
Metastatic tumors to the oral cavity can clinically present as an exophytic mass that tends to favour the posterior dentition. Moreover, patients can present with clinical symptoms of progressive swelling accompanied by paresthesia, pain, and/or trismus. Lesions that are localized to the soft tissues or gingiva only may initially appear to be hyperplastic or inflamed gingiva and can resemble benign lesions such as pyogenic granuloma, peripheral giant cell granuloma, or simply focal fibrous hyperplasia.6 However, with disease progression, other symptoms may include increasing discomfort with mastication, bleeding, super-imposed infection, dysphagia, and eventual disfigurement.4,5 Radiographic examination of metastatic lesions to the bone are often non-specific and can present as osteolytic or mixed radiopaque-radiolucent lesions with a “moth-eaten” appearance with ill-defined margins. The appearance may resemble osteomylelitis, however, malignancy should be ruled out.6 In the face of a diagnostic challenge, an astute clinician should include metastatic disease in the differential diagnosis for any clinically or radiographically detected pathology.
Herein, we report a case of lung adenocarcinoma and a case of colorectal adenocarcinoma to the oral cavity. We stress the importance for primary health care providers and oral health professionals to be wary of metastatic disease to the oral cavity amongst patients with a known cancer history, as well as those with no history of cancer. Due to the clinically challenging nature of diagnosing these lesions, it becomes imperative for clinicians to initiate the proper management plan in order for the patient to receive a definitive diagnosis and begin appropriate treatment as indicated.
Case 1
Fig. 1

Fig. 2

Fig. 3

In November 2017, a 79-year-old gentleman was referred to the Department of Oral and Maxillofacial Surgery at Dalhousie University by his dentist with the chief complaint of “inability to chew and minor bleeding from the lower left”. The patient had been reporting pain from repeatedly biting onto an intra-oral mass during masticatory function.
His past medical history was remarkable for hypertension, coronary artery disease, dyslipidemia, and previous chemoradiation treatment for poorly differentiated adenocarcinoma of right lung originally diagnosed in 2015 (Stage IIIA disease). He remained on disease surveillance via serial computed tomography (CT) scans of the head, chest, and abdomen every three months. His medications included tamsulosin, metoprolol, amlodipine, atorvastatin, and aspirin. He had no drug allergies. His previous surgeries were notable for remote tonsillectomy, pacemaker placement, and two-vessel coronary artery bypass graft. He had quit smoking 25 years ago (with a 17 pack-year history) and admitted to occasional alcohol use.
On clinical examination, the patient appeared systemically well and was in no apparent distress. His vital signs were stable and he was afebrile. He showed no obvious facial asymmetry or extraoral swelling. There was no palpable cervical lymphadenopathy. This lesion was located at the left posterior mandible and appeared as a 3×2 centimeter round and exophytic mass on the buccal aspect of tooth 3.7. The lesion significantly obscured the underlying tooth (Fig. 1) and manipulation of this tooth produced appreciable mobility. The lesion also showed slight hemorrhage with gentle manipulation. The soft tissue adjacent to the lesion and the remainder of his oral examination was unremarkable.
An orthopantomogram was obtained (Fig. 2). Radiographic inspection revealed that tooth 3.7 displayed a “floating” appearance with loss of supporting alveolar bone as well as increased surrounding bone density. There was no appreciable effect on the inferior alveolar neurovascular bundle or adjacent anatomical structures. The remainder of the radiographic exam was unremarkable.
Given the concerning clinical and radiographic presentation along with the patient’s history of malignancy, an incisional biopsy was performed on the day of presentation with local anesthetic and the specimen was submitted for histopathological analysis.
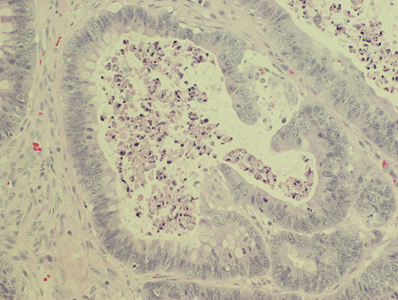
Histologically, the specimen was determined to be consistent with “metastatic adenocarcinoma from a lung primary” (Fig. 3). The biopsy showed a poorly differentiated adenocarcinoma with islands, nests and strands of malignant cells and focal gland formation. The cells exhibited moderate nuclear pleomorphism and there was focal necrosis. The tumor bore a close resemblance to the patient’s known lung primary, diagnosed two years previously.
A new referral was made to the patient’s medical oncologist and an expedited CT scan of the head, chest, and abdomen was obtained. The CT showed enlargement of the lesion in the upper lobe of the right lung along with a new 9 mm metastatic lesion at the right parietal lobe of the brain. With the widespread metastatic nature of the patient’s disease, he and his family made an informed decision to undergo palliative care. Optimal comfort and care was provided to him before he passed away in late February 2018.
Case 2
Fig. 4

Fig. 5

to the photo presented for Case 1 (Figure 2).
Fig. 6
A 71-year-old female presented to the Department of Oral and Maxillofacial Surgery at Dalhousie University with the chief complaint of “pain and swelling of my gums” in her left mandible. She first noticed the swelling in early February 2019, and had subsequently sought medical attention with her general practitioner. At that time, her condition was deemed to be due to a viral illness and was discharged without further management. However, two weeks later, her symptoms increased in severity. This ultimately brought the patient to the emergency department. She was tentatively labeled with “necrotizing ulcerative gingivitis” or “squamous cell carcinoma” and discharged with oral penicillin and topical lidocaine for her symptoms, along with a referral to oral and maxillofacial surgery.
The patient’s past medical history was significant for hypertension, dyslipidemia, stable angina, gastroesophageal reflux disease, and a history of cecal adenocarcinoma (Stage IIA disease) that was surgically treated in 2017 with a right laparoscopic hemicolectomy. In addition, her surgical history included a remote cholecystectomy and bilateral tubal ligation. Her medications included aspirin, atenolol, atorvastatin, rabeprazole, and vitamin B12. She reported a latex allergy and denied any drug allergies. She used alcohol on occasion, had quit smoking 42 years earlier, and denied any recreational drug use.
On clinical examination, the patient was in no apparent distress and denied any dysphagia or airway concerns. She reported that she had felt some relief of her pain when using the medications prescribed by the emergency department one week earlier. She did report some unintended weight loss over the past couple of months and difficulty eating due to pain in her mouth. She did not have any gross facial asymmetry, nor palpable cervical lymphadenopathy. There was unilateral paresthesia on the left aspect of the chin. Intra-oral examination revealed a 2.5 x 2.5 cm exophytic soft tissue mass that surrounded tooth 35 buccally and lingually. The lesion was pink in colour with a white pseudomembrane and a friable texture (Fig. 4).
An orthopantomogram illustrated a normal radiographic exam without any notable bony changes, pathology, or inflammatory concerns in the region of the mass (Fig. 5).
Given the concerning clinical appearance of the lesion combined with the recent history of gastrointestinal malignancy, an incisional biopsy was obtained from the anterior portion of the mass under local anesthetic.
Histologically, the specimen demonstrated evidence of “adenocarcinoma consistent with metastatic colorectal primary.” The sections showed an adenocarcinoma with typical features of colorectal carcinoma, including irregular glands containing necrotic debris admixed with neutrophils (so-called “dirty necrosis”). The overlying surface epithelium was normal. The malignant cells were pseudostratified and elongated and there was focal mucinous differentiation. The tumor bore a close resemblance to the patient’s previous primary cecal adenocarcinoma. Furthermore, immunohistochemistry showed positivity for Cytokeratin 20 and CDX2, with no Cytokeratin 7 positivity. This pattern of expression is typical of colorectal tumors and would be highly unusual for an oral primary (for example of salivary gland origin).
By the time of her follow up at one week after the biopsy, the patient had already displayed stigmata of overt biliary obstruction with clinical jaundice and worsening fatigue. As such, she was immediately transferred to the emergency department for further medical care. At the same time, appropriate interdisciplinary arrangements were made with general surgery, medical oncology, radiation oncology, and palliative care regarding this patient’s ongoing management.
Subsequently, a CT scan of the head, chest, and abdomen was performed revealing extensive disseminated disease throughout her mediastinal, abdominal, and retroperitoneal lymph nodes, along with metastatic lesions in her lungs, liver, pelvis, and brain. Given the extensive and aggressive nature of her condition, the patient opted for palliative care, requesting to enjoy the remainder of her life with her family.
Discussion
It should be emphasized that any type of primary tumor can have the potential for metastasis to the jaws or oral cavity. However, the distribution of the origin of metastatic lesions is different between genders largely because incidences of many cancers are gender-specific. For instance, in men, the most common primary cancers to metastasize to the oral cavity include lung, kidney, liver, and prostate. In contrast for women, the most common primary tumors with oral metastases include breast, genital organs, kidney and colorectal tract.4,6 Also, certain cancers more commonly colonize the mandibular or maxillary skeleton rather than the soft tissues, such as prostate and breast cancer.4,6 In the current report, Case 1 (lung adenocarcinoma) appeared to involve both soft tissue and bone, based on radiographic findings, whereas for Case 2 (colorectal adenocarcinoma), the metastatic lesion primarily involved the mucosa. Both cases involved the posterior mandible, which is a common site for metastatic tumor deposits. Additionally, the patient in the second case presented with a “numb-chin”, which is characteristic and an ominous clinical sign for tumor metastasis to the jaws.7-9
A question that health care providers must ask themselves during the initial work up of a patient presenting with a suspicious lesion in the oral cavity or facial skeleton is if the lesion appears clinically benign or malignant. If it appears to be malignant, the clinician should next determine if the lesion may be a primary intraoral malignancy such as squamous cell carcinoma, or a malignancy from a metastatic tumor. To differentiate between these two possibilities, a proper medical history and biopsy must be obtained. However, certain histopathological features of primary intraoral malignancies may in fact resemble metastatic lesions. For instance, ductal carcinoma of salivary glands can resemble metastatic breast carcinoma.6 Therefore, further confirmation using histomorphology and immunohistochemistry between the metastatic tumor and the primary lesion becomes helpful in resolving the diagnosis. If a tumor is biopsied as a malignancy without a known primary, then the histological profile should guide part of the workup towards searching for the primary tumor.
Clinically detectable metastatic lesions to the jaws and oral cavity are tremendously rare. However, the actual rates of metastatic lesions to the oral cavity are likely higher as autopsy studies have revealed that up to 16% of carcinoma cases contain malignant deposits to the jaws.10 The average time from diagnosis of a primary tumor to the appearance of an oral metastatic lesion is approximately 40 months, with several isolated cases of up to 10 years.4 Unfortunately, the finding of a metastatic lesion foretells a grave prognosis for patients as the average life expectancy from the time of the oral finding is seven months.4,6
Distant metastasis to the jaws and oral cavity from a primary tumor is explained by a proposed mechanism in which extravasated tumor cells can bypass lung filtration through the valveless venous plexus. An increase in intrathoracic pressure can direct blood flow through this venous plexus from the caval and azygous venous system and ultimately shunt tumor cells to bypass the lungs and seed into tissues of the head and neck region.6,11 However, once in the head and neck, the dissemination of tumor cells into either mucosa or bony structure becomes less understood. For bones, metastatic targets include bone with sites that are rich with active marrow, which is a finding that is commonly lacking in elderly patients except for the posterior mandible in which hematopoetic remnants can exist. As such, the remaining sites of active marrow in more mature patients may be targeted by metastatic cancer cells.6,12 As mentioned earlier, the gingiva is the most common site for metastatic colonization amongst the oral soft tissues, and the attraction of metastatic cells to the gingiva may be driven by the presence of inflammation.5 The source of the inflammation is most likely due to the presence of teeth and periodontal disease, in which chronic inflammation produces cytokines such as interleukin (IL)-1 and tumor necrosis factor (TNF)-α that are known to stimulate metastasis by facilitating angiogenesis and establishment of an extracellular matrix milieu suitable for tumor proliferation.13 Therefore, at the very least, chronic periodontal disease or a state of chronic tissue inflammation may in part facilitate metastatic disease development in the oral cavity, but no studies have yet to show a direct cause. Interestingly, the patient in Case 2 admitted to having a food trap between teeth #36 and #38, and this may be a source of chronic inflammation in this scenario.
Literature reports of metastatic disease to the oral cavity and jaws are few and are largely limited to case reports or case series3,14,15 with a bias towards unusual presentations. As such, there are no evidence-based clinical trials on this disease process. Treatment options for oral metastatic lesions include surgical resection with or without concomitant radiation or chemotherapy, along with consideration for the nature of the primary tumor.16,17 As with the presented cases and most cases reported in the literature, the presence of oral metastases unfortunately represents widespread disease and therefore treatment options are largely limited to palliative options and quality of life measures, depending on the degree of systemic involvement.6,18 Future treatments for metastatic deposits to the jaws may deploy the use of bisphosphonates or bone-targeting therapies.6 Routine radiographic exams may not be able to detect any positive findings in certain instances of metastatic disease to the jaws (as with Case 2). Therefore, advanced imaging techniques such as positron emission tomography (PET) scans may be helpful in detecting cases of early metastatic lesions with the hopes of initiating earlier treatment.6 However, this imaging modality was not performed in either of the above reported cases due to the low index of suspicion for metastatic disease. Therefore, the question lies in the costs, indications, and which patients would be best served by receiving a PET scan to screen for occult metastatic disease. In the mean time, clinicians should practice their due diligence with keen physical examinations and history taking skills in order to detect malignancies as early as possible.
Summary
The cases presented represent excellent examples of how oral health professionals can play a vital role, not just in the early diagnosis of oral cancer, but also in the surveillance of metastatic disease. To achieve this, it becomes important for the oral health professional to be vigilant with their history and physicals and be meticulously thorough with their clinical examinations. As with the second case, clinicians should inquire about “red flag” symptoms including unintentional weight loss, fatigue, fevers, and night sweats. An answer of yes to any of these symptoms should prompt further inquiry. They should also pay careful attention to any tissue or radiographic irregularities regardless of whether the patient has any history of malignancy. If a finding is positive, the proper interdisciplinary team should be organized to develop a comprehensive and prompt treatment plan for the patient.
Oral Health welcomes this original article.
Declaration of Patient Consent: Full consent from both patients was obtained for publication of their case details, radiographs, and photos.
Conflicts of Interest: None declared.
References
- Servato JP, de Paulo LF, de Faria PR, Cardoso SV, Loyola AM: Metastatic tumours to the head and neck: retrospective analysis from a Brazilian tertiary referral centre. Int J Oral Maxillofac Surg 42:1391, 2013
- D’Silva NJ, Summerlin DJ, Cordell KG, Abdelsayed RA, Tomich CE, Hanks CT, Fear D, Meyrowitz S: Metastatic tumors in the jaws: a retrospective study of 114 cases. J Am Dent Assoc 137:1667, 2006
- Hirshberg A, Leibovich P, Buchner A: Metastatic tumors to the jawbones: analysis of 390 cases. J Oral Pathol Med 23:337, 1994
- Hirshberg A, Shnaiderman-Shapiro A, Kaplan I, Berger R: Metastatic tumours to the oral cavity–pathogenesis and analysis of 673 cases. Oral Oncol 44:743, 2008
- Allon I, Pessing A, Kaplan I, Allon DM, Hirshberg A: Metastatic tumors to the gingiva and the presence of teeth as a contributing factor: a literature analysis. J Periodontol 85:132, 2014
- Hirshberg A, Berger R, Allon I, Kaplan I: Metastatic tumors to the jaws and mouth. Head Neck Pathol 8:463, 2014
- Penarrocha Diago M, Bagan Sebastian JV, Alfaro Giner A, Escrig Orenga V: Mental nerve neuropathy in systemic cancer. Report of three cases. Oral Surg Oral Med Oral Pathol 69:48, 1990
- Lossos A, Siegal T: Numb chin syndrome in cancer patients: etiology, response to treatment, and prognostic significance. Neurology 42:1181, 1992
- Gaver A, Polliack G, Pilo R, Hertz M, Kitai E: Orofacial pain and numb chin syndrome as the presenting symptoms of a metastatic prostate cancer. J Postgrad Med 48:283, 2002
- Hashimoto N, Kurihara K, Yamasaki H, Ohba S, Sakai H, Yoshida S: Pathological characteristics of metastatic carcinoma in the human mandible. J Oral Pathol 16:362, 1987
- Cumming J, Hacking N, Fairhurst J, Ackery D, Jenkins JD: Distribution of bony metastases in prostatic carcinoma. Br J Urol 66:411, 1990
- Barnes L: Metastases to the head and neck: an overview. Head Neck Pathol 3:217, 2009
- Bickel M, Axtelius B, Solioz C, Attstrom R: Cytokine gene expression in chronic periodontitis. J Clin Periodontol 28:840, 2001
- Nawale KK, Vyas M, Kane S, Patil A: Metastatic tumors in the jaw bones: A retrospective clinicopathological study of 12 cases at Tertiary Cancer Center. J Oral Maxillofac Pathol 20:252, 2016
- Bodner L, Sion-Vardy N, Geffen DB, Nash M: Metastatic tumors to the jaws: a report of eight new cases. Med Oral Patol Oral Cir Bucal 11:E132, 2006
- Nakamura T, Ishimaru J, Mizui T, Kobayashi A, Toida M, Makita H, Iwata H, Shimokawa K: Osteosarcoma metastatic to the mandible: a case report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 91:452, 2001
- Ost P, Bossi A, Decaestecker K, De Meerleer G, Giannarini G, Karnes RJ, Roach M, 3rd, Briganti A: Metastasis-directed therapy of regional and distant recurrences after curative treatment of prostate cancer: a systematic review of the literature. Eur Urol 67:852, 2015
- Andabak Rogulj A, Tomasovic Loncaric C, Muller D, Blivajs I, Andabak M, Vucicevic Boras V, Sekerija M: Solid malignant metastases in the jaw bones. Br J Oral Maxillofac Surg 56:705, 2018
About The Authors
 Johnson Cheung obtained his DDS from the University of Toronto in 2015 and is currently the 4th-Year Resident at the Oral & Maxillofacial Surgery program at Dalhousie University, Halifax, Nova Scotia.
Johnson Cheung obtained his DDS from the University of Toronto in 2015 and is currently the 4th-Year Resident at the Oral & Maxillofacial Surgery program at Dalhousie University, Halifax, Nova Scotia.
 Nicholas Emanuele obtained his DDS from Dalhousie University in 2016 and is currently the 3rd-Year Resident at the Oral & Maxillofacial Surgery program at Dalhousie University, Halifax, Nova Scotia.
Nicholas Emanuele obtained his DDS from Dalhousie University in 2016 and is currently the 3rd-Year Resident at the Oral & Maxillofacial Surgery program at Dalhousie University, Halifax, Nova Scotia.
 Amy Surh obtained her DDS from SUNY Buffalo School of Dental Medicine in 2007. She completed her residency in Oral and Maxillofacial Surgery in 2012 at St. Joseph’s Regional Medical Center, Seton Hall University. She is currently a fellow at the Oral & Maxillofacial Surgery program at Dalhousie University, Halifax, Nova Scotia.
Amy Surh obtained her DDS from SUNY Buffalo School of Dental Medicine in 2007. She completed her residency in Oral and Maxillofacial Surgery in 2012 at St. Joseph’s Regional Medical Center, Seton Hall University. She is currently a fellow at the Oral & Maxillofacial Surgery program at Dalhousie University, Halifax, Nova Scotia.
 Guy Barzan obtained his DDS from the University of Western Ontario in 2012. He obtained his MD and MSc from Dalhousie University in 2019. He is currently the Chief Resident of the Oral & Maxillofacial Surgery program at Dalhousie University, Halifax, Nova Scotia.
Guy Barzan obtained his DDS from the University of Western Ontario in 2012. He obtained his MD and MSc from Dalhousie University in 2019. He is currently the Chief Resident of the Oral & Maxillofacial Surgery program at Dalhousie University, Halifax, Nova Scotia.
 Martin Bullock is currently a professor in the Department of Pathology at Dalhousie University, Halifax, Nova Scotia.
Martin Bullock is currently a professor in the Department of Pathology at Dalhousie University, Halifax, Nova Scotia.
 Chad Robertson obtained his DDS from the University of Western Ontario in 1996. He obtained his MD, MSc, and completed his residency in Oral & Maxillofacial Surgery at Dalhousie University in 2003. He then completed an oncology fellowship at the University of California, San Francisco in 2004. He is currently an associate professor and the OMFS department chair at Dalhousie University.
Chad Robertson obtained his DDS from the University of Western Ontario in 1996. He obtained his MD, MSc, and completed his residency in Oral & Maxillofacial Surgery at Dalhousie University in 2003. He then completed an oncology fellowship at the University of California, San Francisco in 2004. He is currently an associate professor and the OMFS department chair at Dalhousie University.












