Lifelike aesthetics in prosthodontics has been a moving target since the dawn of modern dentistry. The definition of what meets the contemporary understanding of a clinically acceptable prosthesis has always been dependent upon what is considered possible and practical at the time. To date, the aesthetic restoration of a patient’s missing or damaged dentition has competed with its functional restoration, since the achievement of those two goals has historically been mutually exclusive. Functional success has traditionally superseded aesthetic success from a clinical standpoint, but this prioritization has rarely originated from the patient centred perspective. This balance of function vs. aesthetics has always involved some compromise on the clinician’s part – and tolerance on the patient’s – and choosing the appropriate biomaterial has been a challenging task for the clinician and laboratory.
When the complexity of the case increased, or the patient’s expectations were very high, until very recently the materials and techniques available rarely achieved successful biomimesis: both strength and beauty. New developments in the application of materials science are changing this history of compromise, and the clinician has more tools than ever to help deliver excellence. It’s exciting, yet challenging: the rapid development of several options for strong and aesthetic dental biomaterials has presented the clinician and technician with more choice – and complexity – than has ever existed in restorative dentistry.
Biomaterial decision-making starts with the diagnosis. Factors to consider include the quantity and quality of tooth-structure remaining, whether the tooth is endodontically treated, the available restorative space, arch position, parafunction, and the patient’s occlusion. These factors must be considered before prescription of restoration, because optimal prosthetic reconstruction begins with the accurate preparation and impression making of the substrate dentition for the prescribed material.
The dental materials available for the restoration of single or short span prostheses are non-precious metal (i.e. chrome cobalt or similar), cast full gold, porcelain-fused-to-metal (non-precious or precious), lithium disilicate, porcelain-fused-to-lithium-disilicate, monolithic zirconia, and porcelain-fused-to-zirconia. For single-tooth inlays, onlays, and anterior crowns, high-leucite glass ceramics are also an option. Each has its unique characteristics, applications, and advantages and disadvantages, and a thorough discussion of each material in turn would be useful but more appropriate for longer formats. For the purposes of this article, a comparison of the materials’ suitability for a single or short span prosthesis (both in the posterior and anterior aesthetic zone) according the criteria of clinical precision, aesthetics, probable complications, bonding potential, polishability, complications, and cost will be discussed.
Historically, non-precious alloys have been eschewed in favour of some variation of the “full-gold crown.” There are many alloys available for the casting of non-precious, low-Noble, and high-Noble prostheses, and some controversy remains despite decades of discussion regarding their individual advantages and disadvantages. For our purposes, it is sufficient to know that increasing non-precious content increases the unpredictability of the casting properties of the metal (both the accuracy and density of the casting), and increases the potential for marginal misfit, galvanic and lichenoid reactions, and corrosion (Wassell, Walls & Steele, 2002). Further, the potential for corrosion negatively impacts adjacent soft tissue health when the margins are subgingival, and it is worth emphasizing that as the span length increases, the predictability of dimensional accuracy decreases greatly. These all seem like good reasons not to consider a cast non-precious restoration as a viable alternative to more predictable semi-precious or precious restorations, but there are advantages: a large cost savings is possible with non-precious alloys, and their great strength and rigidity provides advantages when restorative space is limited or long span fixed partial dentures are contemplated (Fig. 1).
Fig. 1
Porcelain-Fused-to-Metal (Non-Precious), teeth 13-16 (LHM Dental Studio Ltd.)

The contemporary application of modern non-precious alloys in fixed prostheses can be cast, milled, or laser sintered for their fabrication. An expert dental laboratory can cast individual crowns and short span prostheses with high marginal accuracies and low porosity through careful technique and spruing. A cast non-precious restoration has the advantage of achieving fine-detail marginal accuracy (i.e. smaller than that achievable with milling due to limitations in bur size) for these small prostheses. The concerns regarding dimensional predictability increase with span length, though; milling of non-precious alloys removes the uncertainty of casting accuracy and variable casting density, and theoretically can improve marginal fit in longer span prostheses when the margin design is appropriate for milling. Laser sintering shows promise but, being a relatively new technology, clinical demonstration of its strengths and weaknesses is sparse at this time. For both milled and laser sintered non-precious restorations, though, consider that the time invested in the digital workflow can exceed that required for a skilled technician to fabricate accurate single or short span prosthesis non-precious castings.
Further, while the challenge of opaquing prior to the application of porcelain veneer and the resultant aesthetic compromises are a problem this material shares in common with all metal-based restorations, arguably this is no worse for cast or milled non-precious materials than for any other low-gold alloy. And, because the non-precious coping can be much thinner than the semi- or precious coping while maintaining the same strength, for the same amount of tooth preparation more of the resultant restorative space may be spent on feldspathic porcelain veneer. Lastly, the extremely hard but extremely smooth surface of the polished non-precious unit is very forgiving to the opposing dentition, and, like gold-based restorations, is appropriate when parafunction is present. It is for these reasons that high-quality cast or milled non-precious restorations remain modern options, depending on patient need.
Full gold restorations have been advocated primarily for three reasons: minimal tooth reduction was required, the marginal accuracy was high and biocompatible with the adjacent tissue in subgingival locations, and wear of the opposing dentition was low. When used for inlays, Type I or Type II gold permitted some degree of burnishing of the margins, yielding in skilled hands a very long-lasting restoration. Type III or Type IV gold is too hard for burnishing, and thus crowns and bridges are subject to the same laboratory–based technical challenges as any other prosthesis (Wassell, Walls & Steele, 2002). The casting accuracy makes this material much easier to use, but this advantage comes at great expense. By way of comparison, a single unit high-precious (68% gold) crown can be CAN$200 more than the equivalent size of non-precious crown. In the balance, while cast gold has a long history of clinical success when executed with excellence, so do other, more modern and less costly alternatives.
Porcelain-fused-to-metal (or ceramo-metal) restorations vary greatly in their potential clinical application. The decision to exclude porcelain from the occluding surfaces and the location of that ceramic finish line can play a significant role in the observed consequences of this type of prosthesis. Low-to-moderate leucite-containing porcelain occlusal contacts can be very abrasive to the opposing dentition – even when the surface remains glazed from the laboratory – and occlusal interferences will chip some portion of this veneering porcelain every time (Kelly, 2004). When trying to protect our patients’ opposing dentition, we should consider the single-unit prosthesis with a metal occlusion that is prevented from excursive contact by the patient’s guidance as the default standard. When multiple units are planned, opposing a variety of substrates, decision-making regarding probable wear become very complex and are beyond the scope of this discussion. Regarding the location of the finish line, porcelain is significantly less plaque-retentive than either gold or non-precious alloys; a porcelain butt margin is generally advised (Kawai & Urano, 2001).
As discussed above, either cast-gold or cast/milled non-precious can form the substrate upon which an aesthetic restoration is based. Cost is the primary differentiator between the two options, assuming expert laboratory execution of the prescription. The primary concern, once marginal accuracy is achieved, is the aesthetics of the prostheses. Generally, the aesthetics achieved can be very good with either prosthesis under these circumstances. Using the conventional reduction guidelines for a 1.0 mm porcelain butt margin in an anterior application of PFM restorations, the following values are worth considering: a non-precious coping requires 0.2-0.3 mm thickness, while a gold coping requires 0.4-0.5 mm depending on alloy (Figs. 2, 3); the opaque layer is 0.2-0.3 mm (Fig. 4); the resultant aesthetic porcelain is at best 0.4-0.6 mm thick with a non-precious coping, and 0.2-0.4 mm with a gold coping (Fig. 5). Understandably, the complete opacity of the metal substrate and the opaquing layer required to mask it prior to porcelain application means that the skilled laboratory technician is working from an opaque baseline with only 0.2-0.6 mm of veneering porcelain to achieve the high standard of aesthetics demanded by today’s patients (Jung et al, 2008).
Fig. 2
Porcelain-Fused-to-Metal (Precious), framework ready for soldering (LHM Dental Studio Ltd.)

Fig. 3
Porcelain-Fused-to-Metal (Precious), framework soldered and abraded (LHM Dental Studio Ltd.)

Fig. 4
Porcelain-Fused-to-Metal (Precious, framework opaqued (LHM Dental Studio Ltd.)

Fig. 5
Porcelain-Fused-to-Metal (Precious) (LHM Dental Studio Ltd.)

High-leucite glass ceramics belong here because of their very good aesthetic properties and their modest but context-specific strength. Increasing leucite content strengthens the resultant ceramic without markedly impacting the aesthetic properties of the outcome. An example of this would be IPS Empress from Ivoclar, which is still very popular despite its age for these reasons (Fig. 6).
Fig. 6
High-Leucite Glass Ceramic, teeth 11, 21 (LHM Dental Studio Ltd.)

Lithium disilicate is a type of glass-ceramic that is translucent enough to be used without veneering porcelain (i.e. monolithic), or cut back with veneering porcelain. One very popular example is IPS e.max from Ivoclar. The product is composed primarily of quartz, lithium dioxide, and alumina, and may be either hot pressed into a mold in a fashion similar to casting with the lost wax technique, or it may be milled from solid blocks via CAD/CAM. Each technique has its unique characteristics. The milled product is manufactured as a solid block of lithium meta-silicate free from the defects that can be incorporated in lab-fabricated glass ceramics, and because it is initially partially sintered lithium meta-silicate, it can be milled with minimal wear of CAM burs prior to sintering to its final lithium disilicate state. Because of this two-stage sintering, however, the crystals of lithium disilicate formed are relatively short at 1.5 μm; the flexural strength of the CAD/CAM e.max is 360 MPa and fracture toughness is 2.25 MPa m0.5. By comparison, the e.max Press version contains longer crystals of 3-6 μm and as a result demonstrates greater flexural strength and fracture toughness of 400 MPa and 2.75 MPa m0.5, respectively. By comparison, veneering porcelain demonstrates approximately 90 MPa of flexural strength (Giordano & McLaren, 2010).
It is also important to note that the correct post-fabrication processing must include polishing of the finished prosthesis. Similar to the glass-ceramics, the final glaze applied to lithium disilicate restorations is only temporary; function rapidly degrades the initial smoothness and the relatively coarse unpolished substructure is exposed. As a result, glazed lithium disilicate wears opposing enamel more than Type III gold (Lee et al, 2014). Polishing of the occluding surfaces of the lithium disilicate prosthesis is thought to significantly reduce this wear, with several studies finding that the wear characteristics of the resultant surface of polished lithium disilicate restorations approaches that of human enamel.
With either fabrication technique, the resultant all-ceramic restoration is available in multiple translucencies and shades, and is highly aesthetic in monolithic form. When a higher degree of internal characterization and/or translucency is required, veneering porcelain can be used selectively, resulting in a majority of the restoration being maintained as monolithic lithium disilicate. The inherent shading and translucence of the substrate makes it possible to employ only very thin layers of porcelain to achieve optimal aesthetics; when compared to the challenges in achieving a similarly high standard using conventional porcelain-fused-to-metal restorations, the differences are marked (Fig. 7).
Fig. 7
Lithium Disilicate, teeth 16-26 (LHM Dental Studio Ltd.)

The last modern dental biomaterial remaining is zirconium oxide, or zirconia. It is considered a polycrystalline ceramic, unique from the various glass ceramics and physically unique from the other previously most common polycrystalline ceramic, alumina. The zirconia crystals are doped with yttrium to create a metastable tetragonal crystalline structure at body temperature. The tetragonal crystal lattice is much stronger and denser than the monoclinic crystal structure normally stable at body temperature. When crack propagation is initiated, the energy concentrated at the crack tip disturbs the metastable tetragonal lattice, causing it to revert to the larger, less strong monoclinic lattice structure. The surrounding tetragonal lattice will not permit the expansion of the less dense monoclinic structure, which then effectively squeezes the crack closed and prevents further propagation. This transformation from tetragonal to monoclinic lattice structures is the reason this process is called transformation toughening, and is unprecedented in dental biomaterial science (Conrad, Seong & Pesun, 2007).
There are a variety of strengths and translucencies of zirconia available on the market. The strongest are very opaque, but can be sufficiently aesthetic for posterior restorations
(Fig. 8). As is always true, the increasing translucency comes at the cost of decreased fractural strengths – yet, even the weakest is approximately double the fractural strength of the strongest pressed lithium disilicate while being similarly translucent (for example, Prettau Anterior Zirconia, with approximately 670MPa flexural strength vs. 390MPa). These strengths are sufficient for predictable restoration of anterior or posterior single units, and their survival rate is equivalent to that of traditional PFM restorations (Sailer et al, 2009). Further, polished zirconia is less abrasive than enamel (Janyavula et al, 2013), dental porcelain (Jung, Lee, & Choi 2013) and glazed zirconia (Stawarczyk, Oxcan, and Schmutz 2013), so that the use of the zirconia substrate as the occluding surface remains both more aesthetic and functional than a similarly designed PFM restoration (Fig. 9).
Fig.8
Monolithic Zirconia, teeth 17-14, 24-27 (LHM Dental Studio Ltd.)

Fig. 9
Porcelain-Fused-to-Zirconia, teeth 13-23 (LHM Dental Studio Ltd.)
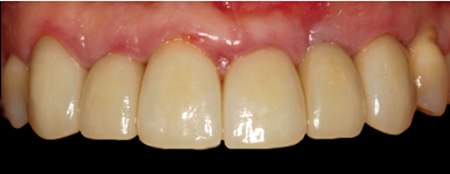
Decision-making between the various materials and techniques available for conventional single and short-span prostheses can be confusing. Predictably adequate structural strengths compromised by predictably inferior aesthetics are hard to justify when so many superior modern alternatives are available to the educated clinician. When aesthetics are important to the patient and clinician, all-ceramic restorations have supplanted conventional metal-based biomaterial options and closely match their functional performance while minimizing the destructive wear associated with occlusal porcelain laminations. Further, because the aesthetic outcomes of these all-ceramic materials are so superior to conventional PFM restorations, experienced clinicians seem to be more conservative in tooth preparation, which has been shown to result significantly fewer all-ceramic prosthesis abutments requiring endodontic treatment vs. PFM abutments over a five-year period (Pjetursson et al, 2007).
Table 1: Biomaterial suitability by intraoral application
 * depending on potential connector dimension (min. 16mm2)
* depending on potential connector dimension (min. 16mm2)
** depending on desired translucence
In conclusion, many clinically acceptable materials are available to meet the patient-specific need for single crown and short-span prostheses (Table 1). Treatment planning must include the pre-operative selection of the appropriate biomaterial to meet the patient’s clinical and aesthetic requirements to ensure the minimal necessary tooth preparation can be selectively applied. OH
Oral Health welcomes this original article.
References
1. Conrad, H. H. J., Seong, W. W., & Pesun, I. J. I. (2007). Current ceramic materials and systems with clinical recommendations : A systematic review. J Prosthet Dent, 98, 389–404.
2. Giordano, R., & McLaren, E. a. (2010). Ceramics overview: classification by microstructure and processing methods. Compendium of Continuing Education in Dentistry, 31(9), 682–697.
3. Janyavula, S., Lawson, N., Cakir, D., Beck, P., Ramp, L. C., & Burgess, J. O. (2013). The wear of polished and glazed zirconia against enamel. The Journal of Prosthetic Dentistry, 109(1), 22–9.
4. Jung, R. E., Holderegger, C., Sailer, I., Khraisat, A., Suter, A., & Hämmerle, C. H. F. (2008). The effect of all-ceramic and porcelain-fused-to-metal restorations on marginal peri-implant soft tissue color: a randomized controlled clinical trial. The International Journal of Periodontics & Restorative Dentistry, 28, 357–365.
5. Jung, Y.-S., Lee, J.-W., Choi, Y.-J., Ahn, J.-S., Shin, S.-W., & Huh, J.-B. (2010). A study on the in-vitro wear of the natural tooth structure by opposing zirconia or dental porcelain. The Journal of Advanced Prosthodontics, 2(3), 111–5.
6. Kawai, K., & Urano, M. (2001). Adherence of plaque components to different restorative materials. Oper Dent; 26, 396–400.
7. Kelly, J. R. (2004). Dental ceramics: current thinking and trends. Dental Clinics of North America, 48(2), 513–30.
8. Lee, A., Swain, M., He, L., & Lyons, K. (2014). Wear behavior of human enamel against lithium disilicate glass ceramic and type III gold. The Journal of Prosthetic Dentistry, 112(6), 1399–405.
9. Passos, S. P., Torrealba, Y., Major, P., Linke, B., Flores-Mir, C., & Nychka, J. a. (2014). In vitro wear behavior of zirconia opposing enamel: a systematic review. Journal of Prosthodontics : Official Journal of the American College of Prosthodontists, 23(8), 593–601.
10. Pjetursson, B. E., Bragger, U., Lang, N. P., & Zwahlen, M. (2007). Comparison of survival and complication rates of tooth-supported fixed dental prostheses (FDPs) and implant-supported FDPs and single crowns (SCs). Clin Oral Implants Res, 18 Suppl 3, 97–113.
11. Sailer, I., Gottnerb, J., Kanelb, S., & Hammerle, C. H. F. (2009). Randomized controlled clinical trial of zirconia-ceramic and metal-ceramic posterior fixed dental prostheses: a 3-year follow-up. The International Journal of Prosthodontics, 22(6), 553–60.
12. Stawarczyk, B., Özcan, M., Schmutz, F., Trottmann, A., Roos, M., & Hämmerle, C. H. F. (2012). Two-body wear of monolithic, veneered and glazed zirconia and their corresponding enamel antagonists. Acta Odontologica Scandinavica, 6357(November 2011), 1–11.
13. Wassell, R. W., Walls, A. W., & Steele, J. G. (2002). Crowns and extra-coronal restorations: materials selection. British Dental Journal, 192(4), 199–202,205–211.
RELATED ARTICLE: Grafting with Biomaterials – Did You Get Consent?
Follow the Oral Health Group on Facebook, Instagram, Twitter and LinkedIn for the latest updates on news, clinical articles, practice management and more!












