Abstract
Peri-implantitis can lead to implant failure. Strategies to reduce risks and treat peri-implantitis can manage implant failure and restore function. These strategies can be derived from information on peri-implantitis progression in patients, including prevalence, diagnostic and microbial findings, the impact of systemic diseases, and peri-implantitis therapies. These reports suggested that: 1) peri-implantitis is higher in patients with periodontitis, smokers, and in patients with implants after 5 years of function; 2) peri-implantitis is microbiologically different from periodontitis; 3) uncontrolled diabetes and cardiovascular disease increase the risk of peri-implantitis; 4) most documented peri-implantitis treatments may be successful, but there is no evidence supporting the most effective peri-implantitis treatment; 5) patients with high risk for peri-implantitis may require more vigilant peri-implant maintenance.
Introduction
Dental implants have become a predictable alternative to fixed and removable partial dentures and is a widely chosen option in restoring full or partial edentulism.1,2 Dental implants have high survival rates of 92.8-97.1% over a 10-year period, and is a desired treatment option for rehabilitating the edentulous patient.3,4 Despite the high implant survival rates, dental implants can be prone to peri-implantitis.5 Peri-implantitis can affect both hard and soft tissues around the osseointegrated implant resulting in bone loss and peri-implant probings and eventually implant failure.6 An overview of the available systematic reviews on peri-implantitis and its management, can be used to devise strategies to reduced risk of peri-implantitis and manage implant failure.
Definition of peri-implantitis
The definition for peri-implantitis has not been consistent for many research studies. The following are the different variations of peri-implantitis definition: 1) the consensus definition of the 1st European Workshop on Periodontology,7 2) inflammation in the peri-implant mucosa indicated as bleeding on probing and/or pus, with supporting bone loss,6 3) a progressive peri-implant marginal bone loss more than 2 mm; and with inflammation indicated by purulence, bleeding on probing, and greater than 6 mm probings, 4) peri-implant probings ≥5mm with bleeding, suppuration, and radiographic bone loss of ≥2.5 mm or ≥ the first three threads,8 5) peri-implant probings >5 mm and bleeding on probing, 6) peri-implant crestal bone loss with inflammation of peri-implant mucosa.9
Recently (2018) the American Academy of Periodontology and the European Society of Periodontology Jointly updated the definition and classification of implant diseases. The designation of early, (Fig. 1) moderate (Fig. 2) and advanced (Fig. 3) peri-implantitis were clearly defined10.
Fig. 1

Fig. 2

Fig. 3

Prevalence and incidence
Prevalence provides information on the proportion of peri-implantitis cases in the population at a given time and indicates how widespread peri-implantitis is. (Table 1) Reported prevalence of subjects with peri-implantitis ranged from 1 – 47% with a mean of 22%.11 Peri-implantitis was less likely in the first 5 years after implant placement, incidence of implants affected by peri-implantitis ranged from 0 – 3.4%.12 After 10 years of implant function, implants affected by peri-implantitis ranged from 5.8 – 16.9%, and patients affected by peri-implantitis ranged from 10.7 – 47.2%.12
Table 1
In addition, higher prevalence of peri-implantitis was reported in smokers.12 Thus, in smokers and patients with dental implants beyond the first 5 years, peri-implantitis was a frequently observed problem.
Incidence provides information on the rate of occurrence of new peri-implantitis cases and indicates the risk of patients with implants developing peri-implantitis. (Table 1) The reported incidence of patients with peri-implantitis was 18.8% and that the incidence of dental implants with peri-implantitis was 9.6%.13 Patients on supportive maintenance programs, the incidence of peri-implantitis dropped to 14.3%.13 Patients who smoke, their incidence of peri-implantitis increased to 36.6%.13 Smokers also have a significantly higher risk of peri-implantitis compared to non-smokers in an implant-based analysis.14
Patients without a history of periodontitis reported significantly lower incidence of peri-implantitis compared to periodontitis patients. (Fig. 4)15-17 Non-periodontitis patients reported a lower incidence of marginal bone loss around implants and peri-implantitis than patients with a history of periodontitis.15 Incidence of peri-implantitis in non-periodontitis patients was observed at 10%, incidence of peri-implantitis was higher in generalized aggressive periodontitis patients at 26%.16 After periodontal treatment, patients with residual probings have more peri-implant sites with peri-implantitis compared to patients without residual probings.17
Fig. 4

Diagnostic findings
Peri-implantitis compared to healthy peri-implant mucosa reported significantly higher IL-1β and TNF-a. (Table 1) However, when peri-implantitis is compared to peri-implant mucositis, IL-1β release was not statistically different. Increased levels of IL-1β and TNF-a in peri-implant crevicular fluid from sites with peri-implantitis have been related to increased gingival index, probing depth, bleeding on probing, and bone loss. In addition, other cytokines IL-4, IL-6, IL-8, IL-10, IL-12, and IL-17, have also been linked to peri-implantitis.18,19
Microbial findings
The microbial findings in peri-implantitis are different from periodontal disease.20 (Table 1) Peri-implantitis sites may include opportunistic microorganisms like gram-negative anaerobic pathogens, gram-positive asaccharolytic anaerobic rods, and Epstein-Barr virus. The following microbes were found to be more prevalent in peri-implantitis compared to peri-implant health: Porphyromonas gingivalis, Aggregatibacter actinomycetemcomitans, Prevotella intermedia, Treponema denticola, human herpesvirus 4 and 5, Epstein-Barr 1, and human cytomegalovirus.21,22 In addition, the following microbes comprised of 30% of the total peri-implantitis microbiota: Porphyromonas gingivalis, Treponema socranskii, Tannerella forsythia, Staphylococcus aureus, Staphylococcus anaerobius, Staphylococcus intermedius, and Streptococcus mitis.21 The peri-implantitis sites have higher mean colony-forming units than healthy sites, and can include opportunistic microbes like Streptococcus mitis, Staphylococcus aureus, Staphylococcus intermedius, and Haemophilus influenzae.21
Effects of systemic disease
Patients with diabetes were at a higher risk of peri-implantitis.23 (Table 1)The probing depths, gingival index, and bone loss were higher in poorly controlled than well-controlled diabetics. However, for type 2 diabetes, the data remain inconclusive.
Patients with cardiovascular disease were at a higher risk for peri-implantitis. (Table 1 ) In addition, these patients with peri-implantitis were 3 times more likely to harbor Epstein-Barr virus.24 For patients with rheumatoid arthritis, statistical evaluation showed no associations.24
Treatment of peri-implantitis
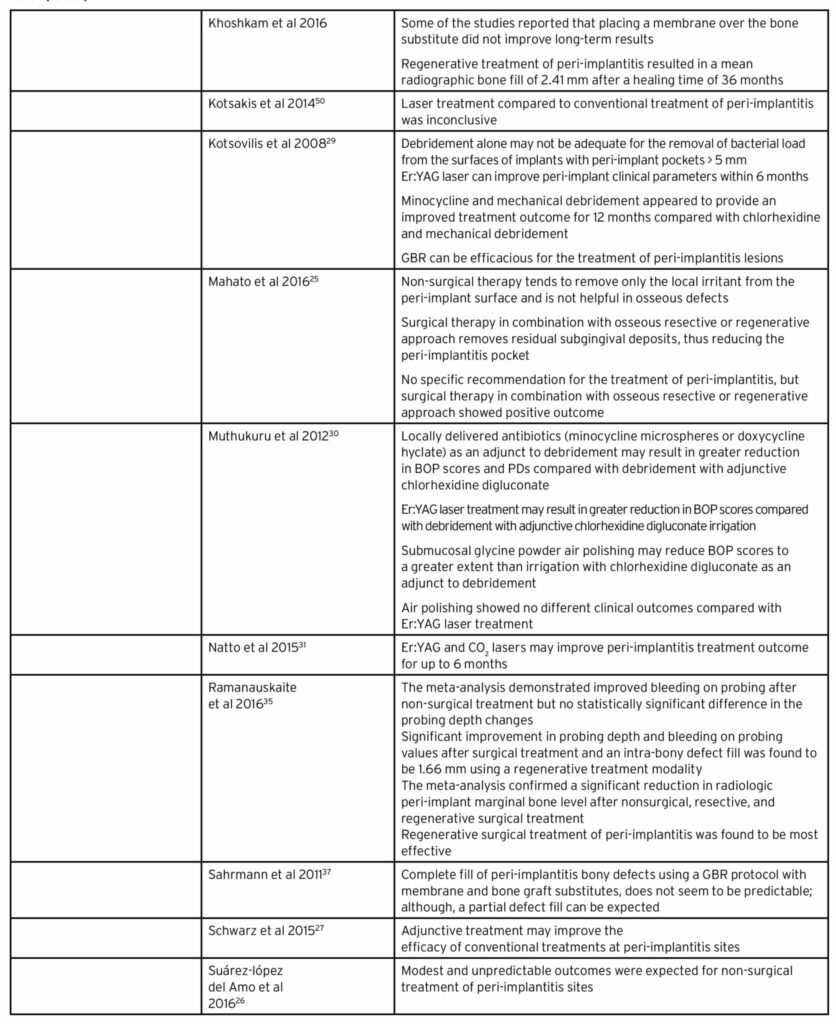
Treatment of peri-implantitis can be non-surgical or surgical. (Table 1) Non-surgical treatment is focused on detoxifying and cleaning implant surfaces, with or without the use of an anti-microbial medicaments. Non-surgical treatments may include manual debridement or ultrasonic debridement, use of chlorhexidine, air-abrasion, local or systemic antibiotics, use of local antiseptics, lasers, and host modulation. Non-surgical therapy can effectively remove local irritants in peri-implantitis sites, but is not as effective in osseous defects.25,26
Surgical treatment is focused on flap elevation, cleaning and detoxifying implant surfaces, with or without anti-microbial therapy, and with or without regenerative membranes or grafting materials. The surgical treatments may include 1) open flap debridement with curettes, ultrasonic scalers, rotating instruments, air abrasion, or laser treatment; 2) resective pocket elimination peri-implant surgery and implantoplasty, 3) regenerative techniques with or without membranes (synthetic membranes, resorbable bovine or porcine collagen) in combination with or without bone substitutes (allograft with or without growth factors, autogenous bone, xenografts, hydroxyapatite, or calcium carbonate).
Additional adjunctive therapy may improve the effectiveness of conventional peri-implantitis therapy.27 Debridement with antimicrobial therapy produced the greatest probing depth reduction compared to debridement.28 At 12-months follow-up, mechanical debridement with minocycline improved peri-implantitis treatment outcome when compared to debridement with chlorohexidine.29,30 The erbium: yttrium-aluminum-garnet (Er:YAG) laser and the carbon dioxide (CO2) lasers can improve implant clinical parameters for up to 6 months.29,31 Er:YAG laser treatment may also result in greater reduction in bleeding on probing (BOP) compared with debridement and adjunctive chlorhexidine irrigation. The commonly used methods for surface decontamination, implantoplasty,29,30 or dental lasers may provide equivalent effects when compared to other intervention.32 The use of glycine powder air abrasives may greatly reduce BOP compared to chlorhexidine digluconate irrigation with debridement; and produced similar clinical outcomes as the Er:YAG laser.30 A network meta-analysis of other non-surgical peri-implantitis treatment showed that non-surgical interventions alone or in combination resulted in greater reduction in probing depth compared to debridement alone.28
Surgical interventions can reduce probing depth by 30-50% from baseline.32,33 Although regenerative procedures reported a mean of 2 – 2.17 mm radiographic bone fill;32-35 the use of a guided bone regeneration with membrane and bone graft did not report predictable positive outcomes.32,34,36,37 When all surgical and non-surgical interventions were pooled together, probing depth and clinical attachment levels were more improved with surgical approaches. However, when the surgical and non-surgical interventions were evaluated separately, there was no significant difference between the two interventions.38
The neodymium-doped yttrium aluminium garnet (Nd:YAG) lasers have also been shown to have the potential of rescuing failing dental implants, by incorporating the laser-assisted peri-implantitis protocol (LAPIP). Several case reports were published to demonstrate radiographic bone fill and clinical attachment gain.39 Histologic evidence of Nd:YAG regeneration in periodontitis-affected teeth40-42 may be a parallel clinical outcome to Nd:YAG peri-implantitis rescue.
Peri-implantitis treatment is considered successful if treated implants report a mean probing of less than 5 mm and no progressive bone loss. At 12 months follow-up, Heitz-Mayfield et al.43 reported successful overall peri-implantitis treatment at 76 – 100% of patients and 75 – 93% of implants; their reported surgical and non-surgical intervention included various combinations of adjunctive treatment options.
Clinical implications of research data
Although most of the systematic reviews covered above are of high quality based on their AMSTAR rating;44 their conclusion derived needed to be interpreted with caution, because of the inherent limitation of their included studies. Some of these limitations include: differing study designs and implant systems, lack of standardization for follow up periods and reported outcomes, restriction to the English language, and differing assessment criteria and definition of peri-implantitis used for the included articles. Other limitations pertaining to peri-implantitis treatment include: heterogeneity in case selection, study design, and treatment; low numbers of studies leading to weak statistical analysis and high risk of bias; and no gold standard for the treatment of peri-implantitis.
For most systematic reviews, there is always a need for more randomized controlled trials. However, despite the limitations of these systematic reviews, systematic reviews are an accumulation of the studies we have available now. As for ongoing clinical situations, these are the only available evidence the clinician can use to derive trends and make an educated decisions to manage their patients with peri-implantitis.
Conclusion
Based on the available published data from systematic reviews, the following are suggested:
- There was a higher incidence of peri-implantitis after 5 years of implant function, and in patients with aggressive periodontitis, chronic periodontitis or a history of periodontitis.
- Smokers are at higher risk for peri-implantitis.
- TNF-a and IL-1β release were significantly elevated in peri-implantitis.
- The microorganisms active in peri-implantitis, is not limited to only periodontopathic pathogens, is different from periodontitis, and may involve some opportunistic pathogens like include Aggregatibacter actinomycetemcomitans, Porphyromonas gingivalis, Prevotella intermedia, Tannerella forsythia, Treponema denticola, Treponema socranskii, Staphylococcus aureus, Staphylococcus anaerobius, Staphylococcus intermedius, Streptococcus mitis, human herpesvirus 4 and 5, Epstein-Barr 1, and human cytomegalovirus 2.
- Systemic conditions including uncontrolled diabetes, and cardiovascular disease have a higher risk of peri-implantitis.
- Surgical treatment of peri-implantitis can reduce probing depths, but guided bone regeneration (GBR) can be unpredictable.
- Different combinations of adjunctive treatments for surgical and non-surgical interventions can produce successful peri-implantitis treatment outcomes that are superior to debridement alone.
- More randomized controlled trials are needed to investigate the most effective treatment intervention for peri-implantitis.
- Post-implant maintenance may be crucial for the reduction of peri-implantitis in high-risk patients.
Oral Health welcomes this original article.
Corresponding author: Miriam Ting, DMD, MS. 250 W Lancaster Ave, Suite 215, Paoli, PA 19312.
Tel: (610) 601-8848, Fax: (412) 365-5261, Email: thinkdentallearninginstitute@gmail.com
Disclosure: MT and JBS have no conflicts of interest with respect to this publication.
References
- Lang NP, Pjetursson BE, Tan K, et al. A systematic review of the survival and complication rates of fixed partial dentures (FPDs) after an observation period of at least 5 years. II. Combined tooth – implant-supported FPDs. Clin Oral Implants Res 2004;15(6):643-53.
- Pjetursson BE, Tan WC, Tan K, et al. A systematic review of the survival and complication rates of resin-bonded bridges after an observation period of at least 5 years. Clin Oral Implants Res 2008;19(2):131-41.
- Albrektsson T, Donos N, Working G. Implant survival and complications. The Third EAO consensus conference 2012. Clin Oral Implants Res 2012;23 Suppl 6:63-5.
- Srinivasan M, Vazquez L, Rieder P, et al. Survival rates of short (6 mm) micro-rough surface implants: a review of literature and meta-analysis. Clin Oral Implants Res 2014;25(5):539-45.
- Klinge B, Meyle J, Working G. Peri-implant tissue destruction. The Third EAO Consensus Conference 2012. Clin Oral Implants Res 2012;23 Suppl 6:108-10.
- Zitzmann NU, Berglundh T. Definition and prevalence of peri-implant diseases. J Clin Periodontol 2008;35(8 Suppl):286-91.
- Albrektsson T, Isidor F, Lang NP, Karring T. Consensus report of Session IV. Proceedings of the 1st European Workshop on Periodontology. London: Quintessence Publishing Co. Ltd. 1994:365-69.
- Ong CT, Ivanovski S, Needleman IG, et al. Systematic review of implant outcomes in treated periodontitis subjects. J Clin Periodontol 2008;35(5):438-62.
- Lang NP, Berglundh T, Working Group 4 of Seventh European Workshop on P. Periimplant diseases: where are we now? – Consensus of the Seventh European Workshop on Periodontology. J Clin Periodontol 2011;38 Suppl 11:178-81.
- Papapanou PN, Sanz M, Buduneli N, et al. Periodontitis: Consensus report of workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J Periodontol 2018;89 Suppl 1:S173-S82.
- Derks J, Tomasi C. Peri-implant health and disease. A systematic review of current epidemiology. J Clin Periodontol 2015;42 Suppl 16:S158-71.
- de Waal YC, van Winkelhoff AJ, Meijer HJ, Raghoebar GM, Winkel EG. Differences in peri-implant conditions between fully and partially edentulous subjects: a systematic review. J Clin Periodontol 2013;40(3):266-86.
- Atieh MA, Alsabeeha NH, Faggion CM, Jr., Duncan WJ. The frequency of peri-implant diseases: a systematic review and meta-analysis. J Periodontol 2013;84(11):1586-98.
- Sgolastra F, Petrucci A, Severino M, Gatto R, Monaco A. Smoking and the risk of peri-implantitis. A systematic review and meta-analysis. Clin Oral Implants Res 2015;26(4):e62-e67.
- Ramanauskaite A, Baseviciene N, Wang HL, Tozum TF. Effect of history of periodontitis on implant success: meta-analysis and systematic review. Implant Dent 2014;23(6):687-96.
- Sousa V, Mardas N, Farias B, et al. A systematic review of implant outcomes in treated periodontitis patients. Clin Oral Implants Res 2016;27(7):787-844.
- Zangrando MS, Damante CA, Sant’Ana AC, et al. Long-term evaluation of periodontal parameters and implant outcomes in periodontally compromised patients: a systematic review. J Periodontol 2015;86(2):201-21.
- Duarte PM, Serrao CR, Miranda TS, et al. Could cytokine levels in the peri-implant crevicular fluid be used to distinguish between healthy implants and implants with peri-implantitis? A systematic review. J Periodontal Res 2016;51(6):689-98.
- Faot F, Nascimento GG, Bielemann AM, et al. Can peri-implant crevicular fluid assist in the diagnosis of peri-implantitis? A systematic review and meta-analysis. J Periodontol 2015;86(5):631-45.
- Rakic M, Grusovin MG, Canullo L. The Microbiologic Profile Associated with Peri-Implantitis in Humans: A Systematic Review. Int J Oral Maxillofac Implants 2016;31(2):359-68.
- Padial-Molina M, Lopez-Martinez J, O’Valle F, Galindo-Moreno P. Microbial Profiles and Detection Techniques in Peri-Implant Diseases: a Systematic Review. J Oral Maxillofac Res 2016;7(3):e10.
- Perez-Chaparro PJ, Duarte PM, Shibli JA, et al. The Current Weight of Evidence of the Microbiologic Profile Associated With Peri-Implantitis: A Systematic Review. J Periodontol 2016;87(11):1295-304.
- Turri A, Rossetti PH, Canullo L, Grusovin MG, Dahlin C. Prevalence of Peri-implantitis in Medically Compromised Patients and Smokers: A Systematic Review. Int J Oral Maxillofac Implants 2016;31(1):111-8.
- Tseng KC, Zheng XY, Qu XH, Lu EY. Risk of peri-implantitis in patients with diabetes mellitus: A meta-analysis. International Journal of Clinical and Experimental Medicine 2016;9(8):15986-95.
- Mahato N, Wu X, Wang L. Management of peri-implantitis: a systematic review, 2010-2015. Springerplus 2016;5:105.
- Suarez-Lopez Del Amo F, Yu SH, Wang HL. Non-Surgical Therapy for Peri-Implant Diseases: a Systematic Review. J Oral Maxillofac Res 2016;7(3):e13.
- Schwarz F, Becker K, Sager M. Efficacy of professionally administered plaque removal with or without adjunctive measures for the treatment of peri-implant mucositis. A systematic review and meta-analysis. J Clin Periodontol 2015;42 Suppl 16:S202-13.
- Faggion CM, Jr., Listl S, Fruhauf N, Chang HJ, Tu YK. A systematic review and Bayesian network meta-analysis of randomized clinical trials on non-surgical treatments for peri-implantitis. J Clin Periodontol 2014;41(10):1015-25.
- Kotsovilis S, Karoussis IK, Trianti M, Fourmousis I. Therapy of peri-implantitis: a systematic review. J Clin Periodontol 2008;35(7):621-9.
- Muthukuru M, Zainvi A, Esplugues EO, Flemmig TF. Non-surgical therapy for the management of peri-implantitis: a systematic review. Clin Oral Implants Res 2012;23 Suppl 6:77-83.
- Natto ZS, Aladmawy M, Levi PA, Jr., Wang HL. Comparison of the efficacy of different types of lasers for the treatment of peri-implantitis: a systematic review. Int J Oral Maxillofac Implants 2015;30(2):338-45.
- Chan HL, Lin GH, Suarez F, MacEachern M, Wang HL. Surgical management of peri-implantitis: a systematic review and meta-analysis of treatment outcomes. J Periodontol 2014;85(8):1027-41.
- Khoshkam V, Chan HL, Lin GH, et al. Reconstructive procedures for treating peri-implantitis: a systematic review. J Dent Res 2013;92(12 Suppl):131S-8S.
- Khoshkam V, Suarez-Lopez Del Amo F, Monje A, et al. Long-term Radiographic and Clinical Outcomes of Regenerative Approach for Treating Peri-implantitis: A Systematic Review and Meta-analysis. Int J Oral Maxillofac Implants 2016;31(6):1303-10.
- Ramanauskaite A, Daugela P, Juodzbalys G. Treatment of peri-implantitis: Meta-analysis of findings in a systematic literature review and novel protocol proposal. Quintessence Int 2016;47(5):379-93.
- Daugela P, Cicciu M, Saulacic N. Surgical Regenerative Treatments for Peri-Implantitis: Meta-analysis of Recent Findings in a Systematic Literature Review. J Oral Maxillofac Res 2016;7(3):e15.
- Sahrmann P, Attin T, Schmidlin PR. Regenerative treatment of peri-implantitis using bone substitutes and membrane: a systematic review. Clin Implant Dent Relat Res 2011;13(1):46-57.
- Faggion CM, Jr., Chambrone L, Listl S, Tu YK. Network meta-analysis for evaluating interventions in implant dentistry: the case of peri-implantitis treatment. Clin Implant Dent Relat Res 2013;15(4):576-88.
- Suzuki JB. Salvaging Implants With an Nd:YAG Laser: A Novel Approach to a Growing Problem. Compend Contin Educ Dent 2015;36(10):756-61.
- Nevins ML, Camelo M, Schupbach P, et al. Human clinical and histologic evaluation of laser-assisted new attachment procedure. Int J Periodontics Restorative Dent 2012;32(5):497-507.
- Yukna RA, Carr RL, Evans GH. Histologic evaluation of an Nd:YAG laser-assisted new attachment procedure in humans. Int J Periodontics Restorative Dent 2007;27(6):577-87.
- Ting M, Huynh BH, Devine SM, Braid SM, Suzuki JB. Laser Treatment of Periodontal Disease: A Systematic Review of Histological Outcomes. EC Dental Science 2018;17.8:1344-67.
- Heitz-Mayfield LJ, Mombelli A. The therapy of peri-implantitis: a systematic review. Int J Oral Maxillofac Implants 2014;29 Suppl:325-45.
- Ting M, Craig J, Balkin BE, Suzuki JB. Peri-implantitis: a comprehensive overview of systematic reviews. J Oral Implantol 2018;44(3):225-47.
- Monje A, Aranda L, Diaz KT, et al. Impact of Maintenance Therapy for the Prevention of Peri-implant Diseases: A Systematic Review and Meta-analysis. J Dent Res 2016;95(4):372-9.
- Sgolastra F, Petrucci A, Severino M, Gatto R, Monaco A. Smoking and the risk of peri-implantitis. A systematic review and meta-analysis. Clin Oral Implants Res 2015;26(4):e62-7.
- Sousa V, Mardas N, Farias B, et al. A systematic review of implant outcomes in treated periodontitis patients. Clin Oral Implants Res 2015.
- Esposito M, Grusovin MG, Worthington HV. Interventions for replacing missing teeth: treatment of peri-implantitis. Cochrane database of systematic reviews (Online) 2012;1:CD004970.
- Graziani F, Figuero E, Herrera D. Systematic review of quality of reporting, outcome measurements and methods to study efficacy of preventive and therapeutic approaches to peri-implant diseases. J Clin Periodontol 2012;39 Suppl 12:224-44.
- Kotsakis GA, Konstantinidis I, Karoussis IK, Ma X, Chu H. Systematic review and meta-analysis of the effect of various laser wavelengths in the treatment of peri-implantitis. J Periodontol 2014;85(9):1203-13.
About the Authors
 Miriam Ting, Director, Think Dental Learning Institute, Paoli, PA; Attending Physician, General Dental Practice Residency, Einstein Medical Center, Philadelphia, PA; and Private Practice, Paoli, PA.
Miriam Ting, Director, Think Dental Learning Institute, Paoli, PA; Attending Physician, General Dental Practice Residency, Einstein Medical Center, Philadelphia, PA; and Private Practice, Paoli, PA.
 Jon B. Suzuki, Clinical Professor, University of Maryland School of Dentistry, Baltimore, MD; Clinical Professor, University of Washington School of Dentistry, Seattle, WA; Clinical Professor, Nova Southeastern University College of Dental Medicine, Fort Lauderdale, FL; and Professor (Emeritus), Temple University, Philadelphia, PA.
Jon B. Suzuki, Clinical Professor, University of Maryland School of Dentistry, Baltimore, MD; Clinical Professor, University of Washington School of Dentistry, Seattle, WA; Clinical Professor, Nova Southeastern University College of Dental Medicine, Fort Lauderdale, FL; and Professor (Emeritus), Temple University, Philadelphia, PA.
RELATED ARTICLE: Prognosis of Implants in Previously Failed Surgical Sites