Abstract
Immediately following tooth extraction, a sequence of events takes place that often results in both alveolar ridge resorption (loss of volume and geometry) and a soft tissue defect. This can impact the correct 3D positioning of the implant when placed at a subsequent treatment with long term functional and aesthetic success affected. Thus, procedures such socket preservation can be employed to preserve the future implant site and various graft materials can be used for this purpose. Surgical re-entry and/or histopathology is considered the gold standard to assess the graft’s healing. The aim of this case report is to document the use of a large particle size (1.2-1.7µm) xenograft for socket preservation. After 5 months of socket graft healing, histopathological core sampling revealed good osteoconduction of the graft and an implant could be placed into the previously deficient site.
Introduction:
Implant therapy is now considered as the best option to replace a missing tooth. The availability of sufficient volume of bone and favorable architecture of the alveolar ridge is a prerequisite to obtain ideal functional and esthetic reconstruction following implant therapy. This however may be compromised due to alveolar bone resorption pattern that occurs after extraction.1
Hard and soft tissue remodeling begins immediately after tooth extraction. Approximately, 2/3 of alveolar ridge resorption occurs in the first 3 months following extraction and nearly 50% of the alveolar ridge is lost over a12 month period. A greater loss in width has been reported as compared to the height, minimizing this bone-resorptive process seems logical, especially if implant placement is planned at a subsequent time.2,3 The primary purpose of socket preservation is preserving the extraction socket dimensions and the avoidance for future extensive surgical procedures to regain the ridge’s height and width for implant placement saving time and energy.4 Studies suggest that socket preservation at extraction time is one technique that can minimize ridge resorption and preserve ridge dimensions.5
Various graft materials are available and have been advocated. Selection is based on operator preferences that are often guided by the size of the defect being grafted and the time between graft placement and when the implant will be placed. Larger defects take longer to fill and larger particle materials have a overall slower resorption rate, allowing host replacement of the graft particles while maintaining the area preventing soft tissue ingrowth. With regards either autogenous, allograft or xenograft materials these are available as cortical, cancellous or a mixture of the two densities. Cortical bone particles, being denser take longer to resorb and are suggested when implant placement will be delayed for over 1 year allowing preservation of the osseous dimensions longer. Cancellous bone, being less dense and more porous is ideally suited when implant placement will be performed between 4 and 6 months. A combination of cortical-cancellous may be indicated for those sites that will have implant placement between 6 months and 1 year after graft placement into the socket. For the purposes of the case presented a large particle xenograft consisting of cancellous particles was selected as the graft material of choice.
Ti-Oss (Ti-Oss, Gyeonggido, Korea) is cancellous bone without any cortical bone with a multiporous structure, a particle size of 1.2-1.7µm, maximizing blood vessel ingrowth. (Fig. 1) A low-temperature processing technique allows ideal, natural surface topography, similar to human bone, stimulating osteoblast activity. A pre HA structure, octacalcium phosphate crystal is found on the surface of Ti-oss®, resulting in fast bone formation during healing. (Fig. 2)
Fig. 1

Fig. 2

Case History:
A 47 year old male reported to the dental office with a non-restorable lower first molar. (Fig. 3) The patient was healthy and reported no significant medical history. After a thorough exam, a delayed implant placement was advised, consisting of extraction of the failing tooth with simultaneous socket grafting and a delayed implant placement. Under patients consent atraumatic extraction was performed under local anesthetic. A vertical incision was made at the mesial aspect of the socket and a full thickness flap was elevated on the buccal, then the socket was thoroughly debrided of granulation tissue, using bone curettes, and rinsed with sterile saline. Dehiscence was noted on the buccal at both the mesial and distal roots to approximately the mid root level. (Fig. 4) Once the site was clean of debris, socket preservation was performed with Ti-Oss xenograft particles placed into the socket and allowed to hydrate by host blood at the site. (Fig. 5)
Fig. 3
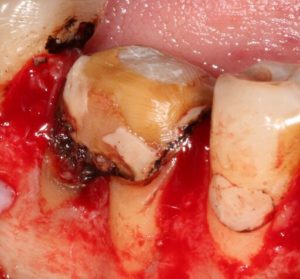
Fig. 4
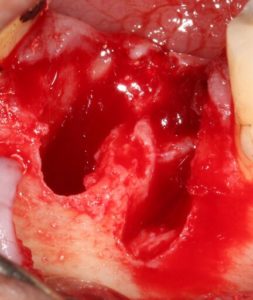
Fig. 5

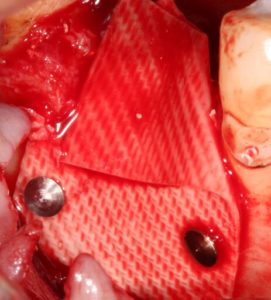
After complete fill of the socket was achieved, a resorbable collagen membrane (Osteogenics Biomedical, Lubbock, TX) was placed over the socket graft material and secured to the buccal with two titanium tacks. (Fig. 6) The flap was repositioned and secured with 3-0 PTFE non-resorbable sutures (Cytoplast suture, Osteogenics Biomedical, Lubbock, TX) and covered with a composite bandage to protect the small area at the crest with membrane exposed. The patient was dismissed and appointed for suture removal two weeks later.
Fig. 6
Postoperative healing was uneventful, and the site was gradually covered by newly formed keratinized soft tissue with no loss of bone graft particles. After 21 weeks (1 sigma), clinically the area was completely covered with newly formed keratinized epithelium, with preservation of the volume and architecture of the ridge. A radiograph was taken to access graft maturation and socket filling. (Fig. 7) The socket graft had a granular appearance radiographically and was reentered to observe healing histologically. Local anesthetic was administered and a crestal incision was made and full thickness flap elevated without vertical releasing incision to expose the bone and previously placed graft at the crest. A bone core was taken with a trephine of inner diameter 2.3mm outer diameter 2.5mm. (Fig. 8) The core was harvested from the center of the site where the implant placement was planned and sent for histological analysis. The site was further prepared to accept an implant 5mm x 10mm (Top DM, Bioner, Madrid, Spain) which was inserted at 40 Ncm. A periapical radiograph was taken to document the implant placement in relation to the graft and adjacent anatomy. (Fig. 9)
Fig. 7
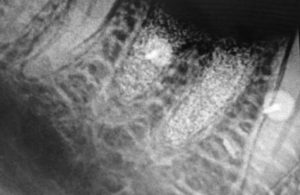
Fig. 8
Fig. 9

The patient returned for restoration of the integrated implant three (3) months later. The cover screw was uncovered, impression taken and healing abutment placed. A custom abutment and crown was returned from the lab and placed into the implant following healing abutment removal. a radiograph was taken to verify full seating of the restorative components. (Fig. 10)
Fig. 10
Histological preparation of specimen
The trephine biopsy was submitted for histological examination was fixed in a formaldehyde fixative (10% neutral buffered formalin) for 24 hours. The specimen was then washed in running tap water for 20 minutes, to ensure complete removal of the fixative solution from the tissue specimen and then immersed in 20ml of a weak organic acid (5% formic acid) to decalcify the specimen for microscopic analysis. The decalcifying solution was maintained at room temperature (18°-30°) and at varying pH gradients. The decalcification status was checked using a Calcium Oxalate chemical test. After the decalcification step, acid from the surface of tissue was neutralized by immersing the decalcified bone into a 5-10% aqueous sodium bicarbonate solution for several hours. The specimen was further processed and dehydrated in increasing grades of alcohol (50% to 100%) followed by treatment in xylene to remove the alcohol from the tissue. This was processed further by placement in liquid paraffin overnight to replace the xylene and permanently infiltrate the wax in the tissue specimen. The tissue specimen was then oriented properly in a mold and filled with liquid paraffin. A plastic cassette was placed over the mold and allowed to cool. The sectioning of the specimen was done using a microtome (Leica RM 2125RT). Sections of 3 to 4 micron thickness were taken and the ribbon of the sections was picked up with a glass slide and deparaffinized after adequate drying using xylene to the remove the paraffin wax. Decreasing grades of alcohol were then used to remove xylene and rehydrate the tissue to prepare it for staining. Freshly prepared Ehrlich’s haematoxylin solution was used for staining. The acid differentiation was shortened to one or two fast dips in 0.5% acid alcohol. The staining time for eosin solutions (0.5-1%) was shortened to 30 seconds. The slides were observed under research
microscope (Olympus BX 53) and photomicrographs were clicked using Olympus EPL 3 camera.
Results:
The submitted H & E stained sections of the trephine core biopsy was composed of predominantly mature lamellated bone with a few remnants of the graft material. (Fig. 11) The mature bone demonstrated basophilic cement lines, osteoblastic rimming and osteocytes within osteocytic lacunae. (Fig. 12) The periphery of the section shows remnants of graft material surrounded by a fibroclleular connective tissue stroma. (Fig. 13) New bone formation in a fibrocellular background stroma was noted. (Fig. 14)
Fig. 11
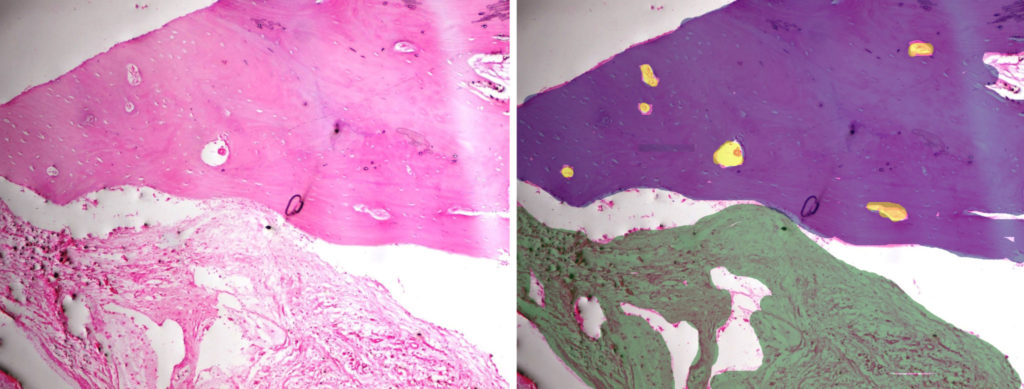
Fig. 12
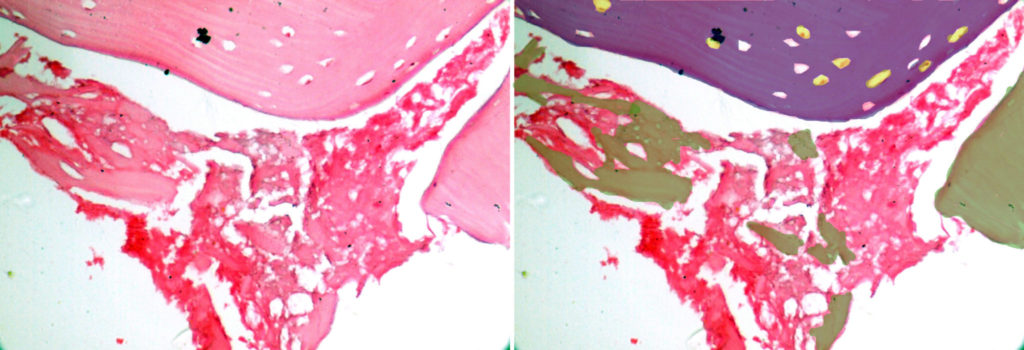
Fig. 13
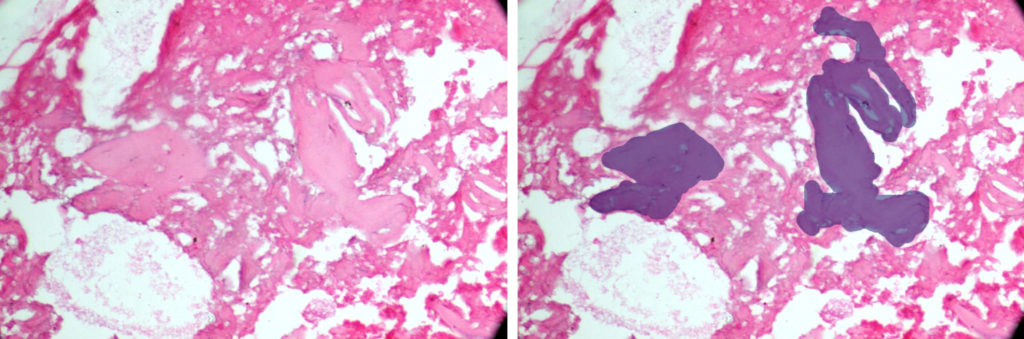
Fig. 14
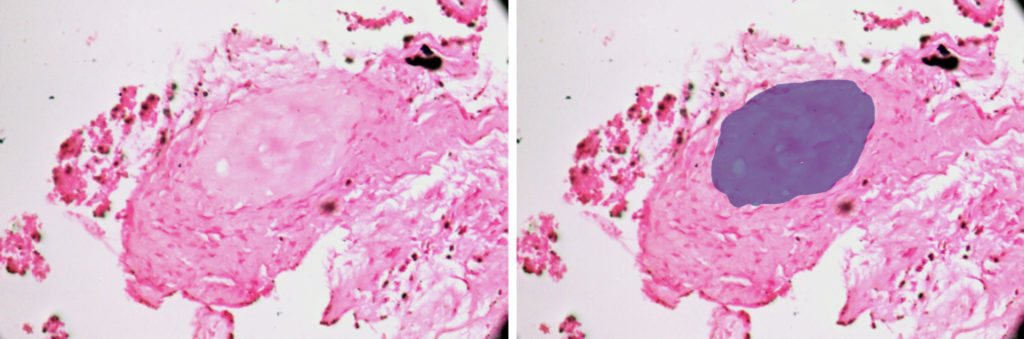
Discussion:
In the present case, the augmented site was covered with a resorbable membrane and overlaid by a composite bandage to prevent food lodgment and protect the site during the initial healing period. Primary closure of the site was not possible as the flap can not be advanced at a molar extraction site sufficiently, hence a membrane is indicated to cover the graft material. This approach was selected in order to minimize patient morbidity, surgical time, and also to avoid displacement of the mucogingival junction. It is assumed that Full thickness flap elevation and advancement for covering the socket will protect the grafting material. However, it is been proved in literature that achieving primary closure does not present beneficial effects on preserving the ridge width. On the contrary it leads to esthetic problems due to coronal displacement of the buccal keratinized gingiva. In addition, patients experience more discomfort, also altering the soft tissue profile of the site and distortion of the vestibule negatively effects the health status of the supporting tissues around dental implants.6
Although autogenous bone is considered as the gold standard for many osseous regeneration procedures. It, however leads to morbidity of the donor site and limitations in the amount of bone available are reported.7 Recently, some investigators reported using a xenogenic graft for socket preservation. Xenograft have been referred to as having osteoconductive capabilities, and to present a slow resorption rate that may contribute to maintain tissue volume.8 Thus, for the present case the authors used a xenograft which is a deproteinized bovine bone mineral (DBBM) with a particle size of 1.2-1.7µm. Nevins et al, reported decreased alveolar bone height loss in extraction socket augmented with DBBM.9 It was closed with a resorbable collagen membrane and then covered with a composite bandage for wound protection.
Alveolar ridge preservation can be considered successful, when it not only preserves the contour of the alveolar ridge externally, but also histologically the type of bone formed through socket preservation is of superior quality and quantity.10 To assess the quality of bone formation, surgical re-entry was done. Out of all the methods available to evaluate regeneration, surgical re-entry into previously surgically treated site is the gold standard as it provides the clinician with the advantage of directly viewing the healing. At the same time a bone sample for histological examination was also obtained, which is the most reliable method to evaluate the progress of regeneration. For this purpose the bone core was taken from where implant was to be placed.
Histological observation of the large particle size xenograft (Ti-Oss) presented, the osteoconductive characteristics allowed apposition of mature vital bone in direct contact with graft material. The newly formed bone was characterized by the presence of osteocytes in the inner part, as well as osteoblastic and osteoclastic activity in the outer part. These findings indicate that Ti-OSS may be suitable for bone augmentation procedures due to its osteoconduction characteristics.
Conclusion:
In this case report large particle size xenograft was utilized for alveolar socket preservation, resulting in pronounced regeneration of high-quality bone capable to support implant placement after a 21 week healing period. Socket grafting with the material described allowed preservation of the dimensions of the bone (width and height), while yielding quality bone at the site to accommodate implant placement.
Oral Health welcomes this original article.
References
- Zhnag W, Shen G, Wang X, Yu H, Fan L, Evaluation of alveolar bone grafting using limited cone beam computed tomography.Oral Surg Oral Med Oral Pathol Oral Radiol 2012; 113:542-48.
- Schropp L,Wenzel A , Kostopoulos L & Karring T. Bone healing and soft tissue contour changes following single-tooth extraction: a clinical and radiographic 12- month prospective study. The Int J Periodontics Restorative Dent 2003; 23,313–23.
- Van der Weijden F, Dell’Acqua F, Slot DE. Alveolar bone dimensional changes of post-extraction sockets in humans: a systematic review.J Clin Periodontol. 2009; 36:1048–58.
- Irinakis T .Rationale for Socket Preservation after Extraction of a Single-Rooted Tooth when Planning for Future Implant Placement.J Can Dent Assoc 2006; 72:917–22.
- Willenbacher M, Al-Nawas B, Berres M, Kämmerer PW, Schiegnitz E. The effects of alveolar ridge preservation: a meta-analysis. Clin Implant Dent Relat Res. 2016; 18:1248–68.
- Fairbairn P, Leventis M, Mangham C, Horowitz R .Case Report Alveolar Ridge Preservation Using a Novel Synthetic Grafting Material: A Case with Two-Year Follow-Up. Case Rep Dent 2018; 1-8.
- Jambhekar S., Kernen F., Bidra A. S. Clinical and histological outcomes of socket grafting after flapless tooth extraction: a systematic review of randomized controlled clinical trials. J Prosthet Dent. 2015; 113:371–82.
- Mahesh L, Narayan TV, Bali P, Shukla S socket preservation with alloplast: discussion and a descriptive case. J Contemp Dent Pract 2012; 13:934-37.
- Nevins M, Camelo M, De Paoli S, Friedland B, Schenk RK, Parma-Benfenati S et al . .A study of the fate of the buccal wall of extraction sockets of teeth with prominent roots. Int J Perio Rest Dent. 2006; 26:19–29.
- Horváth A, Mardas N, Mezzomo L A, Needleman IG, Donos N.et al. Alveolar ridge preservation. A systematic review. Clin Oral Invest 2013; 17:341-63.
 Lanka Mahesh is in private practice in Delhi, India. His practice is solely focused on oral implantology. Drlanka.mahesh@gmail.com
Lanka Mahesh is in private practice in Delhi, India. His practice is solely focused on oral implantology. Drlanka.mahesh@gmail.com
 Gregori Kurtzman is in private general dental practice in Silver Spring, Maryland, USA and a former Assistant Clinical Professor at University of Maryland in the department of Restorative Dentistry and Endodontics and a former AAID Implant Maxi-Course assistant program director at Howard University College of Dentistry. He has lectured internationally on the topics of restorative dentistry, endodontics and implant surgery and prosthetics, removable and fixed prosthetics, and periodontics, and has over 700 published articles globally. dr_kurtzman@maryland-implants.com
Gregori Kurtzman is in private general dental practice in Silver Spring, Maryland, USA and a former Assistant Clinical Professor at University of Maryland in the department of Restorative Dentistry and Endodontics and a former AAID Implant Maxi-Course assistant program director at Howard University College of Dentistry. He has lectured internationally on the topics of restorative dentistry, endodontics and implant surgery and prosthetics, removable and fixed prosthetics, and periodontics, and has over 700 published articles globally. dr_kurtzman@maryland-implants.com
 Meenu Taneja Bhasin completed her BDS from K.L.E Institute of Dental Science Belgaum, and MDS in Department of Periodontics & Oral Implantology from C.O.D.S, Davangere, Karnataka. She completed her basic and advanced implant training from Dentsply, Germany in 2009 and her MDS in Oral Implantology from College Extra-Universitaire D’Implantologie Orale et Maxillo-Faciale, France 2019. Currently she is in private practice at Dr. Bhasin’s Super-Speciality Dental Clinic, Delhi.
Meenu Taneja Bhasin completed her BDS from K.L.E Institute of Dental Science Belgaum, and MDS in Department of Periodontics & Oral Implantology from C.O.D.S, Davangere, Karnataka. She completed her basic and advanced implant training from Dentsply, Germany in 2009 and her MDS in Oral Implantology from College Extra-Universitaire D’Implantologie Orale et Maxillo-Faciale, France 2019. Currently she is in private practice at Dr. Bhasin’s Super-Speciality Dental Clinic, Delhi.
Saurabh Juneja is a reader in the department of Oral Pathology at ITS Dental College in UP, Ghaziabag, India.
To view the entire Oral Surgery 2020 issue, please click here.












