
The Problem
Every week there will likely be a patient in your office with a white, red, or white & red mucosal patch in their oral cavity. It is estimated that 1-5% of the population, at any given time, may harbour one such lesion.1 With few exceptions, the oral mucosa should be pink. Some of these lesions will unfortunately transform into oral squamous cell carcinoma (OSSC), the most common form of oral cancer. Will it be your patient? Could your patient potentially have oral pre-cancer, properly termed oral potentially malignant disorder (OPMD)? What action should you take?
Initial Management
The management of this patient is not as clear cut as you might think, even for very experienced and highly trained clinicians.
One decision is an absolute certainty. If there is no obvious cause for any oral lesion, it should be excised completely and submitted for a histopathology diagnosis. If the lesion is too large for complete excision, an incisional biopsy or multiple incisional biopsies should be performed of the most clinically abnormal or representative area(s). When doing any biopsy, try to include some neighbouring, visibly normal tissue. If you are not skilled enough to perform the surgery, the patient should be referred to someone who is confident. In the case of OPMD, someone who sees these patients on a regular basis is ideal since the long-term management of these patients can be complex and frustrating.
When an oral lesion seems to be caused by a sharp tooth, broken down filling, or loose denture, the cause should be treated, and the patient reassessed a few weeks later to ensure the lesion has resolved. If it has not, a biopsy should be considered. Where a pre-existing mucosal disease is present, (i.e., lichen planus) biopsy may be reserved for instances where a new observation is superimposed on the initial disease.
The Need to Biopsy
Why biopsy? In a meta-analysis including 1,956 patients with biopsy proven OPMD or OSCC, experienced dental clinicians were asked to speculate on the pathology results, prior to the biopsy, based solely on the clinical exam. They were able to identify that a lesion was in fact present in 93% of cases, which is reassuring. However, when providing a diagnosis based solely on the clinical examination of the patient, clinicians did very poorly (diagnostic odds ratio = 6.1), which is not improved by diagnostic adjunctive light devices.2 An incorrect diagnosis, based on clinical assessment alone could delay definitive treatment and have dire consequences. Suspicious lesions must be biopsied or excised completely! Tissue(s) must be submitted for histopathology assessment. Leave it to the Oral Pathologist to provide a definitive histopathology diagnosis.
Oral Potentially Malignant Disorders
There are dozens of possible diagnoses for the white, red, or red and white lesions we often encounter. While referred to as leukoplakia, erythroplakia and mixed lesions, these terms are only descriptors, not diagnoses. These terms provide little insight into underlying pathologic processes, and in no way do they predict future behaviour. Some lesions may be indolent, but others may be pre-cancerous or even malignant. As oral healthcare providers, we are often the first to identify OSCC or OPMD in our patients. Be vigilant, thorough, and respond appropriately, in a timely manner.
There are a number of oral diseases that may undergo malignant transformation. The WHO, recognizing the serious potential of these lesions collectively grouped them in 2005 under the banner of OPMD. In large meta-analysis studies, the overall malignant transformation of OPMD lesions was found to be 7.9 – 12.1%.3,4 OPMD diagnoses includes lichenoid mucositis, oral epithelial dysplasia, submucous fibrosis, chronic hyperplastic candidiasis, and lichen planus, among others. The malignant transformation rate across these diagnoses can range from 1.4 to a high 49.5 %.3
Table 1: OPMD
| Leukoplakia | Proliferative Verrucous Leukoplakia |
| Erythroplakia | Oral Submucous Fibrosis |
| Oral Lichen Planus | Actinic Keratosis ( Cheilitis ) |
| Lichenoid Mucositis | Oral Lupus Erythematosus |
| Dyskeratosis Congenita | Palatal Lesion of Reverse Smoking |
| Oral Graft vs Host Disease |
Dysplasia and Dysplasia Grading
The majority of OPMD patients will show some degree of oral dysplasia, either as a sole diagnosis, or in addition to an overall diagnosis of perhaps hyperkeratosis or lichen planus. Dysplasia, by definition, is abnormal growth.
When issuing their report, pathologists usually attempt to describe the degree of the abnormality of the tissue to the surgeon; they grade dysplasia. How bizarre does the tissue appear to them? What is their subjective feeling about the future behaviour of the tissue?
Currently, oral pathologists use the WHO three-tiered system of mild, moderate, and severe dysplasia to classify tissue.5 This system was initially proposed in 1967, with the last revision in 2017. Dysplasia grading is based on cellular abnormalities as well as aberrations in normal epithelial architecture.
Pathologists have tried for years to objectify some of the characteristics of dysplasia grading to increase the reproducibility of grading. If 2 oral pathologists look at the same slide, will they always come to the same grading conclusions? Many classification schemes have been proposed.1,6 The creation of so many systems reflect the frustrations oral pathologists have experienced trying to develop an evaluation which is truly reproducible.
Dysplasia grading is inherently a subjective assessment. Studies have demonstrated the poor reproducibility of dysplasia grading not just in oral mucosa, but also other organ tissues. In one study, it was shown that if oral pathologists were asked to examine an oral mucosal biopsy showing some degree of dysplasia, and again look at the exact same slide 3 months later, they agreed with themselves only 46-60% of the time!7 In a study by Speight, 2 oral pathologists looking at 846 cases of OPMD with dysplasia agreed with each other only 69.9% of the time, and if a 3rd pathologist looks at the same cases, they could not come to a consensus in 7.3% of cases.8 Opponents of the WHO 3-tiered system have proposed a 2-tier system arguing that it has better reproducibility, but evidence for this is inconclusive.9
The poor reproducibility of dysplasia grading prompted prominent Indian oral pathologist Ranaganathan to write, “[professional licensing bodies] should take steps to provide external quality assessment in reporting OED (oral epithelial dysplasia) among oral and general pathologists.”10
In an essay by Bosman titled “Dysplasia classification: pathology in disgrace”, he suggests that when pathologists are reporting, there should be a dysplasia diagnosis called “I am not sure”. He states that it is entirely artificial to impose subjective grading categories on a process which is a continuum. Bosman adds that “a new gold standard based on molecular parameters correlated with clinical outcomes is needed.”11
Dysplasia Grading As a Predictor of Malignant Transformation
Why be concerned about the degree of dysplasia in the first place? Is severe dysplasia worse than mild dysplasia, or is dysplasia just dysplasia?
There is an assumed implication that the higher the grade of dysplasia, the more likely the tissue is going to transform into cancer. This is not always true. One might also surmise that mild dysplasia progresses to moderate dysplasia, and on to severe dysplasia, then to OSCC. Evidence for this is not strong.12 Some dysplastic lesions may evolve to a lesser degree of dysplasia or even revert back to a normal appearance.
Does the degree of dysplasia grading correlate well with the risk of malignant transformation? There are many published studies looking at this relationship. A 2015 review and meta-analysis showed that higher dysplasia grading is associated with a higher risk of OSCC, but mild and moderate lesions can all progress to cancer.13 In a study of 1,888 leukoplakia lesions, Chaturvedi found that 39.6% of lesions that transformed to cancer had no dysplasia at all.14 Dost in his study and long term follow up of 338 OPMD patients came to the final conclusion, “[Dysplasia Grading] cannot be used reliably as a guide for treatment decision making.”14
The Role of Dysplasia Grading in OPMD Patient Management
Oral dysplasia grading is a subjective test with poor reproducibility and poor predictive value, but it remains the “Gold Standard” for predicting malignant transformation in OPMD patients. Why is it that we continue to use dysplasia grading in practice?
Sound guidance for the care of patients with OPMD continues to elude clinicians. Some investigators have tried to bolster the predictive ability of dysplasia grading by incorporating dysplasia grading into complex patient management algorithms. Dysplasia grading is combined with patient demographics and the clinical features of the OPMD lesion, in hopes of enhancing predictive performance. The most notable schemes would be the Liverpool Algorithm15 and the Speight Algorithm.16 While age, lesion size and lesion location are objective measures in these algorithms, the subjective nature of oral dysplasia grading inherently compromises the performance of these guides.
The ability to develop effective management strategies for OPMD patients is also compromised by difficulties in comparing studies and data sets from different cohorts around the globe, due to the absence of standardized patient registries and uniform follow up parameters.17,18
The Need For An Objective Predictor of Malignancy
Most oral cancers arise from OPMD lesions. The necessity for an objective test, rather than subjective, to predict which OPMD patient’s lesion might progress to OSCC should now be obvious. Let’s stop guessing who these progressors might be. The critical need for a reliable molecular marker predictor is discussed at length in an editorial by Edwards, where he addresses the frustration in managing the treatment of OPMD patients, especially those with mild dysplasia.19
Objective and accurate identification of those patients truly at high risk for OSCC would more effectively direct surveillance, which should ultimately lead to either interrupting OSCC development, or to earlier cancer diagnosis.
Oral cancer is a very common malignancy worldwide. It is a very deadly disease with 1 person in North America dying of the disease every 45 minutes. This is largely because only 25% of patients present with early-stage disease.20 In the most medically advanced countries, the mortality associated with OSCC has changed little over the last 50 years and their 5 year survival remains near a disappointing 64%, while outcomes for many other cancers have shown dramatic improvement.20 OSCC is also one of the most expensive diseases to treat, with an estimated median 1st year cost in the U.S. of $212,929 (2017 USD) placing a tremendous burden on health care systems.21
Many Oral cancer patients never return to productive life after survival of their OSCC treatment, further compromising the economy and society. Mass screening for OSCC, in hopes of identifying lesions at an early stage, has never been shown to be cost effective, except in high risk populations.22 Who are these high risk patients?
Researchers are trying to identify a molecular test to objectively and accurately identify high risk OPMD patients. A review of these molecular efforts is summarized in a paper by Su.23 One of the most promising markers for malignant transformation that Su discusses is S100A7.
S100A7 Bioprotein As A Predictive Marker For Oral Cancer In OPMD Patients
S100A7 is a calcium binding protein that is found in high concentration in the malignant cells of many cancers and is associated with poor outcomes.24 S100A7 is found both in the nucleus and cytoplasm of malignant cells, where it affects many cellular processes ultimately leading to uncontrolled cellular division as well as cellular invasion.25 Dey et al., studying the P38/MARK and RAB2A pathways concluded S100A7 is the “major contributing factor in the occurrence of OSCC.”26
High concentration of S100A7 has been associated with poor outcomes in patients known to have OSCC regardless of the treatment used. In addition, Tripathi also observed increasing concentrations of S100A7 in abnormal oral mucosa (some with dysplasia), but also in squamous hyperplasia without dysplasia.27 This suggested subcellular carcinogenic changes could be identified using an S100A7 assay prior to the morphologic changes seen in dysplasia.
In an effort to identify a potentially accurate predictive marker for OSSC in OPMD patients, researchers identified 811 candidate tissue markers using iTRAQ chromatography.28 The candidates were narrowed down to 5 biomarkers using immunohistochemical (IHC) staining, and as suspected, S100A7 was identified as the best performer.29
Based on a cohort of 150 cases of OPMD at Mount Sinai Hospital (Toronto, Ontario), with known clinical outcomes, S100A7 and cell morphology were digitally imaged and assessed to generate a completely objective 5 year oral cancer risk curve and risk assessment tool for OPMD patients.29,30 The potential value of a quantitative biomarker-based risk score (qBRS) – S100A7 testing – in clinical practice is discussed by Darling.30
Clinical Use of S100A7 qBRS Testing
How does the S100A7 qBRS testing work?
Conventional histopathology is performed on the patient’s biopsy. If the diagnosis is OPMD, the patient and clinician are warned of the increased OSCC potential. To perform S100A7 testing, more unstained microscopic slides are cut from the patient’s original biopsy specimen. These slides are treated with a tagged anti-human S100A7 antibody. The images of the IHC stained microscopic slides are then digitized. The slides are analyzed using AI-assisted software to quantify cellular S100A7 and evaluate cellular morphology. The entire analysis is objective in nature.
The qBRS score of the patient’s test is then compared to a large ongoing cohort of patients that have been followed for many years with known cancer or non-cancer outcomes. An individualized 5-year cancer risk can then be rendered to the patient.
Better testing should mean better guidance and ultimately better clinical outcomes.
Consider the following 2 cases. They demonstrate the frustration in management of OPMD patients and the potential role of the objective predictive, S100A7 qBRS test.
Case #1
Case 1: Initial Presentation. Biopsy: “ulceration and inflammation“
| Time 0 | She noticed a tender lesion on the right side of her tongue that had been present for a few weeks. The patient had a chronic bruxism history and wore a nighttime bite guard. She admitted to occasionally biting her tongue during sleep. On examination, she had a 3 – 4 mm ulceration on the left side of her tongue. (Fig. 1) There also were areas in her mouth suggestive of mild lichen planus. The lesion looked like a traumatic ulcer, so patient was advised to have her bite guard trimmed and return in a few weeks. |
| 3 months | The “sore” still had not resolved. Reluctant to have the lesion excised, she sought other options. Betamethasone, 1.4 mg was injected into the lesion, and she was placed on a lichen planus mouth rinse. Over the next few months, this treatment led to a reduction. |
| 9 months Surgery #1 13 months | The lesion had still not resolved completely. While the lesion had no obvious clinical features of malignancy, the persistence of the ulcer was disturbing. The lesion was excised. The histopathology assessment showed the following: “ulceration, inflammation, and cellular atypia [that] may represent a reactive change at the margin of the lesion….. close follow-up of this area with removal of all sources of irritation is recommended”. There was no mention of dysplasia (OPMD), and thankfully malignancy. |
| 13 months | The lesion recurred 4 months after excision. It was now 5 mm in diameter, slightly white, with a very small central ulcer. Continued observation was selected by the patient. Patient was lost to follow-up. |
| 2 years Surgery #2 | The patient returned and the recurrent lesion identified at last visit was resected with 5 mm of healthy surrounding tissue. (Fig. 2) The histopathology this time was squamous cell carcinoma. According to the pathology report, the margins, deep and lateral, were clear of tumour. She was referred to a Regional Cancer Care Hospital (RCCH) for consultation. No further treatment was advised by them at that time. |
| 5 years 3 months Surgery #3 and #4 | Three years after her original cancer diagnosis, she had another lesion at the same location. The RCCH staff performed a small biopsy which showed “at least moderate dysplasia”. A larger excision was performed 1 month later which showed “dysplasia only”. |
| 7 years | She is discharged from the RCCH follow-up surveillance. |
| 8 years 10 months Surgery #5 | It was now 9 years after her first complaints of a “sore”, and she returned to private practice care. She had a new white lesion in the exact same location as her first. The lesion is excised again with healthy margins. The pathologist reported “moderate to severe dysplasia”. |
| 9 years 4 months Surgery #6 | She developed another new lesion in the same location. It was immediately resected. This one showed “high grade dysplasia”. |
| 11 years 9 months | Her tongue appeared to be clinically normal for the 2.5 years since last visit. Regular follow-up continues. |
This patient is a very healthy, 46-year-old, female, dental hygienist. She is a non-smoker and rarely drinks alcohol.
This case demonstrates the following:
- Experienced clinicians have difficulties making a correct diagnosis from clinical examination only.
- Classic histopathology diagnosis is often of limited value in directing management of OPMD.
- The “Field Effect of Cancerization”, described decades ago, was vividly displayed in this case.31 Patients with OPMD are prone to recurrent lesions and even with wide resection margins, the recurrence rate may approach 50% with average time to recurrence of 1.3 years.32
- OPMD patients need to be monitored with some frequency for at least 5 years after initial presentation, but how closely? Dysplasia grading offers little guidance.
- The management of OPMD can be very frustrating for both the patient and clinician. Removing OPMD tissue, reduces, but does not eliminate risk of OSCC.33
- OPMD is difficult to eradicate.
Fig. 1
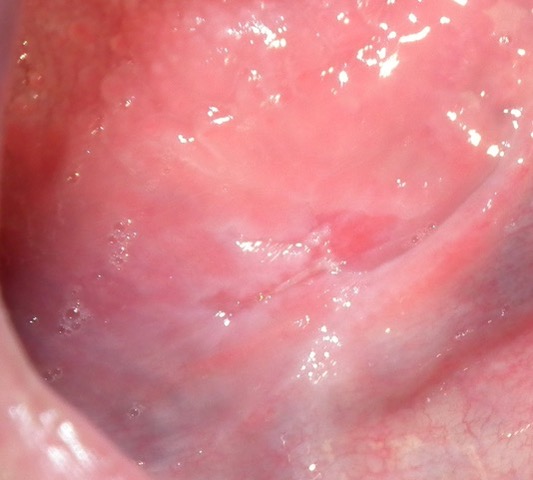
Fig. 2

Case #2
| Time 0 Surgery #1 | She was referred for consultation regarding a white patch on her left lateral border of the tongue, present for less than 6 months. There was no apparent cause, so the lesion was excised. The clinical diagnosis was hyperkeratosis, rule out OPMD. (Fig. 3) The pathology report showed “chronic lichenoid mucositis with mild epithelial dysplasia”. An S100A7 qBRS assessment was done on the original biopsy tissue as part of an observational research study.34 The personalized S100A7 qBRS for this patient was elevated, and her 5-year risk was estimated to be 37%. (Fig. 4) She agreed to be followed for a minimum of 5 years. |
| 5 months Surgery #2 | There was a small recurrence 4 x 3 mm at the anterior resection margin. This tissue was removed with a margin of healthy, apparently normal tissue, and was found to show “hyperkeratosis with mild dysplasia”. S100A7 qBRS testing was performed which showed an elevated 5-year cancer risk of 61%. (Fig. 5) Follow-up every 3 months continued. |
| 21 months | There was a second recurrence, again at the exact same location as the original lesion. This lesion was only 3 mm in diameter, slightly white, with a surrounding reddish area. There seemed to be a tiny central ulcer. The patient thought she may be biting her tongue in the area. She thought if her bite guard was adjusted, it might help. |
| 30 months Surgery #3 | The ulcer was not apparent, which was somewhat reassuring, but there was now a larger area of mixed leukoplakia measuring 4 x 8 mm. In light of her increasing S100A7 qBRS and the high-risk “mixed” appearance of the lesion, a new larger resection was recommended. A 4 – 5 mm wide, seemingly healthy margin was included. The diagnosis was “well differentiated micro-invasive squamous cell carcinoma to a depth of 0.9 mm… one lateral margin is positive for tumour”. |
| 32 months Surgery #4 | The patient was referred to a RCCH. Respecting the concept of “field cancerization”, a 3 x 3 cm diameter mucosal resection is performed. (Fig. 6) The pathology report showed “2 foci of superficially invasive squamous cell carcinoma, each less than 1 mm diameter with depth of invasion of 0.1 mm; margins well negative”. There is no need for a broader or deeper resection, neck dissection, or radiation treatments. |
| 8 years | After release of the patient to the private practice care, no new lesions have been seen. (Fig. 7) |
At presentation, this patient was a 56-year-old healthy female, except for Graves disease for which she took Synthroid 15 mg OD. She underwent a past tonsillectomy, hysterectomy, and tubal ligation.
Key points from this case:
- Clinical observation is limited to acknowledgement that the tissue is abnormal.
- There are many diagnoses in the OPMD group, including lichenoid mucositis that may undergo malignant transformation.
- Dysplasia does not necessarily progress, while the lesion undergoes malignant transformation, and any grade of dysplasia can transform.
- The degree of dysplasia grading may not correlate with the risk of malignant transformation.
- Apparently healthy margins by clinical examination may harbour residual tumour or dysplastic tissue.
- Early diagnosis and conservative intervention can be curative and eliminate the need for expensive adjunctive treatment services with associated morbidity.
- S100A7 qBRS assessment can be a useful tool in management of OPMD patients.
Initial presentation
Biopsy #1
Fig. 3
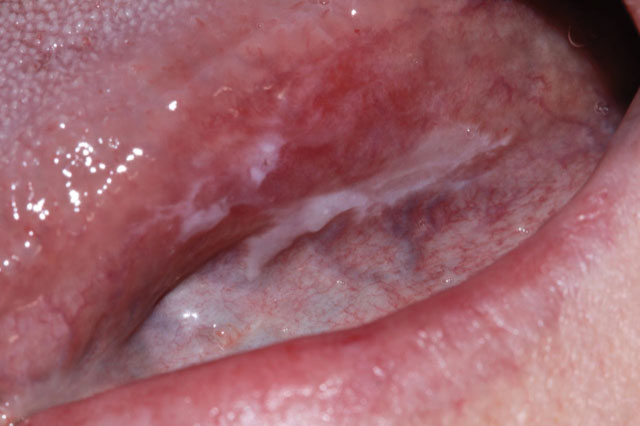
Biopsy #1
Fig. 4

Biopsy #2
Fig. 5
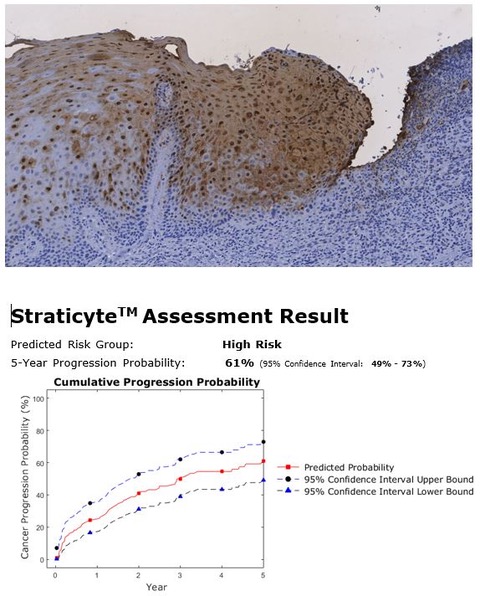
Fig. 6
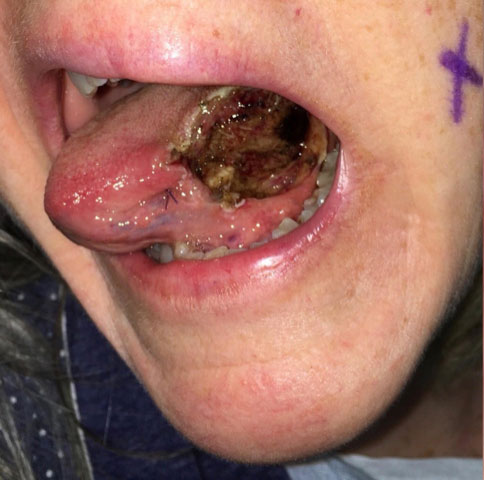
Fig. 7

Discussion
Dentists and dental hygienists are often the first clinicians to recognize that a patient may have OPMD. Most OSCC’s develop from OPMD. There are many diagnoses in the OPMD classification, and they all have the potential to undergo malignant transformation, some more than others. Which patients need to be monitored more carefully should not be a guess.
Early OSCC diagnosis is critical for long term survival. The “Time to Treatment Completion” has 3 components:
- Time to seek treatment.
This is the most significant factor in determining the survival of OSCC patients. This is the longest delay period, and public education has failed to reduce this. - Time from presentation at the family DDS or MD until OSCC is diagnosed.
This is where clinicians can make a difference with better identification of high-risk patients and more timely/appropriate management, including performing a biopsy.35 - Time from diagnosis of OSCC to completion of treatment.
This is usually short. Initiation of surgery and radiotherapy is fast.
Clinicians have been dependent on dysplasia grading for interception of early OSCC’s for the past 50 years. Dysplasia grading has provided limited insight into the potential behaviour of OPMD lesions. Algorithms have attempted to address some of the short comings of dysplasia grading. Pritzker et al, suggests that frequent photographing of oral lesions in OPMD patients, similar to the widespread policy with patients with dysplastic nevus syndrome would be more helpful than dysplasia grading.36 All of these efforts are, however, to some degree, dependent upon subjective observations.
The need for an objective risk assessment predictor for OSCC has never been more apparent, especially as the number of oral cancers continues to rise. Commercially available risk predictors have been used for many years, to manage cancer and “pre-cancer” in other organs. Examples include Afirma, Mammaprint, and Endopredict. Why not Oral Cancer?
The use of S100A7 as a potential predictive biomarker for malignant transformation in OPMD patients has been evaluated in several centres and patient populations. In a recently submitted multi-centered research study, based on long term follow up of 302 patients, the “S100A7 qBRS” was found to have a sensitivity of 96% and a NPV of 95%. This is comparable to the aforementioned commercially available risk assessment tools for other cancers.
One of the strengths of S100A7 qBRS testing, is its superior ability compared to dysplasia grading in predicting outcomes in those OPMD patients with either no dysplasia, or minimal dysplasia; identifying high-risk transforming tissue before the morphological changes of dysplasia are seen.29
S100A7 qBRS testing is not meant to replace dysplasia grading. Conventional histopathology evaluation is still required to determine if OPMD is present, as this is an essential prerequisite for S100A7 qBRS testing.
The S100A7 qBRS testing for OPMD is now commercially available under the trade name Straticyte® from Proteocyte Diagnostics Inc. in Toronto, Canada. For any patients with OPMD, the clinician can request, for a fee, Straticyte testing, by contacting Proteocyte through Patient Care Solutions. A request will be made to the original histopathology lab for unstained slides, Straticyte testing performed, and a report issued to the requesting clinician. Better testing and better care lead to better outcomes.
Oral Health welcomes this original article.
References
- Ranganathan K, Kavitha L. Oral epithelial dysplasia: Classifications and clinical relevance in risk assessment of oral potentially malignant disorders. J Oral Maxillofac Pathol. 2019;23:19-27.
- Epstein JB, Guneri P, Boyacioglu H, Abt E. The limitations of the clinical oral examination in detecting dysplastic oral lesions and oral squamous cell carcinoma. J Am Dent Assoc. 2012;143:1332-1342.
- Iocca O, Sollecito TP, Alawi F, et al. Potentially malignant disorders of the oral cavity and oral dysplasia: A systematic review and meta-analysis of malignant transformation rate by subtype. Head Neck. 2020;42:539-555.
- Mehanna HM, Rattay T, Smith J, McConkey CC. Treatment and follow-up of oral dysplasia – a systematic review and meta-analysis. Head Neck. 2009;31:1600-1609.
- WHO Classification of Tumours. 4th ed. Lyon, France: International Agency for Research on Cancer; 2017.
- Sadiq H, Gupta, P., Singh, N., Thakar, SS., Prabhakar, I., Thakral, J. Various grading systems of the oral epithelial dysplasia: A review. Int J Adv Health Sci. 2015;1:20-26.
- Krishnan L, Karpagaselvi K, Kumarswamy J, Sudheendra US, Santosh KV, Patil A. Inter- and intra-observer variability in three grading systems for oral epithelial dysplasia. J Oral Maxillofac Pathol. 2016;20:261-268.
- Speight PM, Abram TJ, Floriano PN, et al. Interobserver agreement in dysplasia grading: toward an enhanced gold standard for clinical pathology trials. Oral Surg Oral Med Oral Pathol Oral Radiol. 2015;120:474-482 e472.
- de Freitas Silva BS, Batista DCR, de Souza Roriz CF, et al. Binary and WHO dysplasia grading systems for the prediction of malignant transformation of oral leukoplakia and erythroplakia: a systematic review and meta-analysis. Clin Oral Investig. 2021;25:4329-4340.
- Ranganathan K, Kavitha L, Sharada P, et al. Intra-observer and inter-observer variability in two grading systems for oral epithelial dysplasia: A multi-centre study in India. J Oral Pathol Med. 2020;49:948-955.
- Bosman F. Dysplasia classification: pathology in disgrace? J Pathol. 2001;194:143-144.
- Sharma M, Hosmani, JV., Tiwari, V. Epithelial Dysplasia: different grading system and its applications. J Int Oral Health. 2010;2:1-16.
- Shariff JA, Zavras AI. Malignant Transformation Rate in Patients Presenting Oral Epithelial Dysplasia: Systematic Review and Meta-Analysis. Journal of Oral Diseases. 2015;2015:854636.
- Dost F, Le Cao K, Ford PJ, Ades C, Farah CS. Malignant transformation of oral epithelial dysplasia: a real-world evaluation of histopathologic grading. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014;117:343-352.
- Field EA, McCarthy CE, Ho MW, et al. The management of oral epithelial dysplasia: The Liverpool algorithm. Oral Oncol. 2015;51:883-887.
- Speight PM, Khurram SA, Kujan O. Oral potentially malignant disorders: risk of progression to malignancy. Oral Surg Oral Med Oral Pathol Oral Radiol. 2018;125:612-627.
- Baharvand M, Tehranchi, A., Taghavi, N., Rahmani, S., Sadeghi, HMM., Tabrizi, AT., Panahi, MH., Hajighasem, P. Setting up a Registry for Oral Potentially Malignant Disorders (OPMD). Int J Cancer Manag. 2022;15:e115640.
- Saldivia-Siracusa C, Gonzalez-Arriagada WA. Difficulties in the Prognostic Study of Oral Leukoplakia: Standardisation Proposal of Follow-Up Parameters. Front Oral Health. 2021;2:614045.
- Edwards PC. The natural history of oral epithelial dysplasia: perspective on Dost et al. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014;117:263-266.
- Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7-33.
- Pang J, Crawford K, Faraji F, Ramsey C, Kemp A, Califano JA, 3rd. An Analysis of 1-Year Charges for Head and Neck Cancer: Targets for Value-Based Interventions. Otolaryngol Head Neck Surg. 2020;163:546-553.
- Speight PM, Palmer S, Moles DR, et al. The cost-effectiveness of screening for oral cancer in primary care. Health Technol Assess. 2006;10:1-144, iii-iv.
- Su YF, Chen YJ, Tsai FT, et al. Current Insights into Oral Cancer Diagnostics. Diagnostics (Basel). 2021;11.
- Peng G, Tsukamoto S, Okumura K, Ogawa H, Ikeda S, Niyonsaba F. A Pancancer Analysis of the Oncogenic Role of S100 Calcium Binding Protein A7 (S100A7) in Human Tumors. Biology (Basel). 2022;11.
- Qi Z, Li T, Kong F, et al. The Characteristics and Function of S100A7 Induction in Squamous Cell Carcinoma: Heterogeneity, Promotion of Cell Proliferation and Suppression of Differentiation. PLoS One. 2015;10:e0128887.
- Dey KK, Bharti R, Dey G, et al. S100A7 has an oncogenic role in oral squamous cell carcinoma by activating p38/MAPK and RAB2A signaling pathway. Cancer Gene Ther. 2016;23:382-391.
- Tripathi SC, Matta A, Kaur J, et al. Nuclear S100A7 is associated with poor prognosis in head and neck cancer. PLoS One. 2010;5:e11939.
- Ralhan R, Desouza LV, Matta A, et al. Discovery and verification of head-and-neck cancer biomarkers by differential protein expression analysis using iTRAQ labeling, multidimensional liquid chromatography, and tandem mass spectrometry. Mol Cell Proteomics. 2008;7:1162-1173.
- Hwang JT, Gu YR, Shen M, et al. Individualized five-year risk assessment for oral premalignant lesion progression to cancer. Oral Surg Oral Med Oral Pathol Oral Radiol. 2017;123:374-381.
- Darling M, Hassan, A., McLean, L. Psoriasin: A new biomarker in the identification of cancer risk in oral lesions. Oral Health. 2018.
- Slaughter DP, Southwick HW, Smejkal W. Field cancerization in oral stratified squamous epithelium; clinical implications of multicentric origin. Cancer. 1953;6:963-968.
- Sundberg J, Korytowska M, Holmberg E, et al. Recurrence rates after surgical removal of oral leukoplakia-A prospective longitudinal multi-centre study. PLoS One. 2019;14:e0225682.
- Guneri P, Maghami, E., Boyacioglu, H., Ho, AS., Epstein, JB. Outcomes of surgical management of dysplastic oral mucosal lesions versus observation: A systematic analysis. Otorhinolaryngol Head Neck Surg. 2019;4.
- Darling M. Early prediction of oral cancer by S100A7 immunohistochemistry signature-based assessment ClinicalTrials.gov identifier: NCT04622462Updated November 14, 2022.
- Saka-Herran C, Jane-Salas E, Mari-Roig A, Estrugo-Devesa A, Lopez-Lopez J. Time-to-Treatment in Oral Cancer: Causes and Implications for Survival. Cancers (Basel). 2021;13.
- Pritzker K, Darling, MR., Hwang, JTK., Mock, D. Oral Potentially Malignant Disorders (OPMD): What is the clinical utility of dysplasia grade? Expert Review of Molecular Diagnostics. 2021;21:289-298.
About the Authors

Barrie M. Renick, Newmarket Dental Specialists. 641 Davis Dr #200 Newmarket, Ont. L4A 3V8. barrierenick@gmail.com

Jason Hwang, Proteocyte Diagnostics. MaRS Centre South Tower, 101 College St Suite 200. M5G 1L7. jhwang@proteocyte.com

Taylor McGuire, Maxillofacial Surgeon, Division Head – OMFS & Dentistry. The Ottawa Hospital, Children’s Hospital of Eastern Ontario. Argyle Associates. 499 Terry Fox Dr #15, Kanata, Ont K2T 1H7. taylor.mcguire@gmail.com

Tim McGaw, Oral & Maxillofacial Pathologist, Univ. of Alberta, 5-357 Edmonton Clinic Health Academy. 11405 – 87 Ave NW, Edmonton AB, T6G 1C9. tim.mcgaw@ualberta.ca









