
For over 25 years, SciCan Ltd., a Canadian company, has been focused on protecting patients, doctors and healthcare providers with innovative infection control equipment. Through a sustained legacy of growth and expansion, SciCan is now a global leader in infection control specializing in dentistry and ophthalmology in more than 100 countries around the world.
In response to a number of questions which we have received from our Canadian dental providers regarding changes in infection control guidelines, SciCan would like to provide some clarity on the use of Sterilizers in a Dental Practice. All SciCan Sterilizers are Health Canada licensed Medical Devices and designed to allow users to conform to specific requirements of the CSA Standards for reprocessing medical devices in a dental practice.
There are two types of sterilization cycles available in steam sterilizers; Gravity and Dynamic Air Removal. Steam Flush Pressure-Pulse (SFPP) and Pre-Vacuum are two modes of Dynamic Air Removal technologies used for sterilization. Both deliver validated steam sterilization cycles that provide efficacious sterilization when used according to the Instructions for Use (IFU). More specifically, the STATIM® Units use Dynamic Air Removal Steam Sterilization, by the method of SFPP technology, and the BRAVO™ Units use Dynamic Air Removal Steam Sterilization, by the method of Pre-Vacuum.
Several Public Health Associations and Dental Regulatory Authorities across Canada have put together guidelines and checklists to be used as a guide during inspections of a dental office, which assess alignment with best practices for infection prevention and control. To help you understand how SciCan sterilizers meet these requirements we have included an example from Ontario using the Checklist:
Reprocessing in Dental Practice Settings1
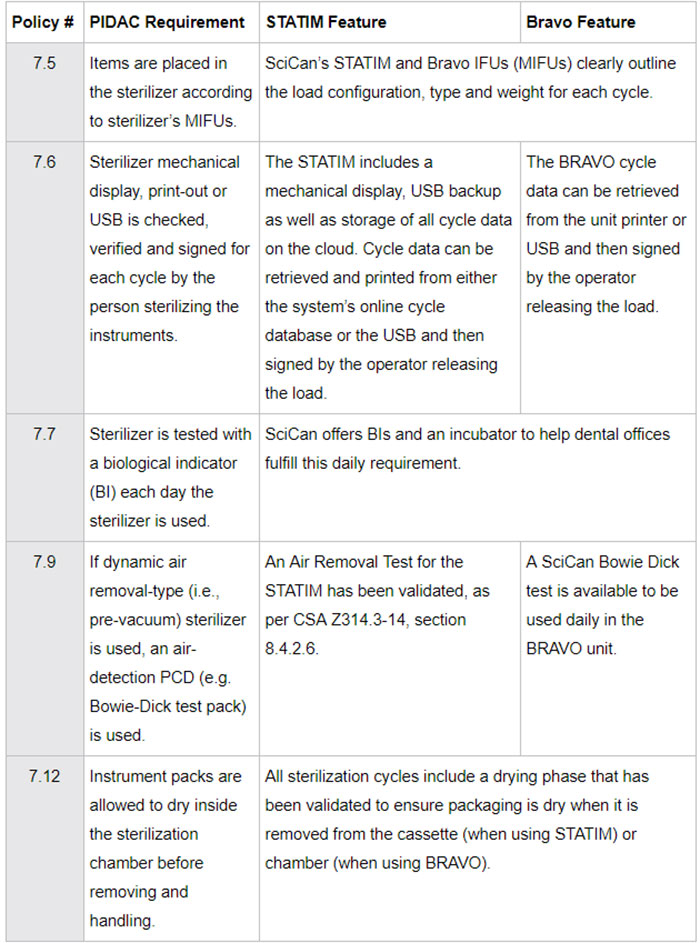
In summary, the use of STATIM and BRAVO Sterilizers allow users to conform to the specific requirements of the CSA Standards for reprocessing medical devices in a dental practice.
SciCan is dedicated to providing evidence-based Infection Control recommendations, best practices and the most innovative and effective infection control equipment.
Additional information pertaining to sterilization requirements and recommendations that are important to a dental clinic can be found at http://www.scicancanada.ca/faq. Should you have any questions or concerns that aren’t addressed here, please don’t hesitate to contact our Customer Service team at 800-667-7733 or customerservice@scican.com.
Kindest Regards,













