Introduction
Peri-implantitis is a high prevalence, biofilm-initiated pathological condition characterized by inflammation of the peri-implant mucosa with progressive loss of supporting bone surrounding a functional endosseous implant.1-3 If left untreated, peri-implantitis can lead to loss of osseointegration and ultimately, implant failure.
Current therapeutic approaches for peri-implantitis include non-surgical and surgical treatments followed by ongoing supportive therapy.4 As with treatment of periodontal diseases, initial phase therapy for peri-implantitis typically involves non-surgical debridement; adjunctive use of locally delivered or systemtic antibiotics can also be considered.5 While this oftentimes leads to improvement of peri-implant mucosal health and reduction of inflammation, evidence from controlled clinical studies has shown that the potentially beneficial clinical outcomes obtained from non-surgical therapy are typically limited to a period of 6-12 months.6 Surgical treatment of peri-implantitis can be resective and/or regenerative depending on the anatomical and morphological features of the resultant peri-implant bone defect(s).7 Various regenerative treatments for peri-implantitis, which include the use of bone grafts/bone substitutes with and without barrier membranes (mostly resorbable collagen), have been evaluated and have demonstrated improved and stable long-term (> 3 years) clinical and radiographic outcomes.8,9 Unfortunately however, there is still little consensus regarding optimal methods for predictably inducing regeneration of bone lost due to peri-implantitis.
Over the past decade, several studies have described the use of dehydrated human de-epithelialized human amnion-chorion membranes (ddACM) for various surgical procedures in dentistry.10,12 ddACM is derived from human placental tissue which is considered to be non-immunogenic, and thus can be transplanted to non-identical recipients with minimal rejection-related inflammation at the surgical sites.13 In addition to its barrier function, ddACM has also been shown to be a carrier of numerous (> 250) biological factors which are known to play an important role in wound healing including, but not limited to, various extracellular matrix molecules, growth factors, and anti-inflammatory cytokines.14 Furthermore, ddACM has also been shown to have antimicrobial properties against pathogenic oral anaerobes.15 Taken together, it is likely that these features promote accelerated healing thus making them advantageous for the regenerative surgical treatment of peri-implantitis.
There is currently a paucity of evidence with respect to the use of ddACM as part of a regenerative strategy for the surgical treatment of peri-implantitis. Described below is a case of peri-implantitis treated with a regenerative approach involving guided bone regeneration (GBR) using bovine-derived xenograft particulate bone (BDX) and ddACM with 2-year clinical and radiographic follow-up.
Clinical Presentation
A 47-year-old healthy male patient was referred for consultation regarding bone loss and infection affecting the implant in the #11 site. The implant was placed and restored abroad in the patient’s native country and had been in function for 3 years at the time of referral. The affected implant was cement-retained and splinted to the crown of the adjacent implant occupying the #21 site (which was screw retained). The patient was asymptomatic but aware of the reason for referral. On examination, the implant was not mobile and the peri-implant gingival tissues appeared erythematous and edematous. Probing depths (PD) along the buccal aspect of the implant ranged from 8-10 mm with bleeding on probing (BOP) and pus was easily expressed from the peri-implant gingival crevice of the buccal tissues upon palpation. Peri-apical radiograph revealed moderate depth angular bone loss at the mesial aspect of the #11 implant associated with a radiopaque entity on the implant surface. (Fig. 1) Based on the clinical and radiographic findings, a diagnosis of peri-implantitis was made for the 1.1 implant.
Fig. 1

Case Management
Initial therapy included non-surgical debridement along with adjunctive systemic antibiotics (amoxicillin 500 mg three times daily for 1 week) which led only to minimal improvement in peri-implant soft tissue appearance but without reductions in PD or BOP and suppuration observed at re-evaluation 6 weeks later. Various treatment options were discussed with the patient including continued non-surgical maintenance, regenerative surgical therapy, as well as implant removal and site reconstruction for future implant placement. The patient wanted to retain the implant and elected for surgical regenerative therapy. Informed consent was obtained to proceed with the surgical treatment.
Under local anaesthesia, a full thickness mucoperiosteal flap was elevated on the buccal aspect of the #11 implant with oblique vertical releasing incisions at the mesio-buccal and disto-buccal line angles. Upon flap elevation, granulation tissue and a calcified surface deposit was observed on the implant surface. (Fig. 2) The implant surface was thoroughly and carefully debrided with periodontal curettes. Upon closer examination of the calcified material, it had the appearance of retained cement. (Fig. 3) The residual bony defect was characterized by a substantial dehiscence of the buccal bone with concomitant exposure of ~ 5 threads. (Fig. 4) The peri-implant defect morphology was consistent with a Class 1b/Grade A defect7 given that the implant remained within the envelope of bone and had intact palatal bone. The implant crown was left in place. Implant surface detoxification was carried out first by chemical rinsing of the implant surface with 3% H202 (Fig. 5) and was then supplemented with the use of a titanium brush (i-Brush; Hubermed, Canada) in a slow speed handpiece (900 rpm) under copious irrigation as previously described by de Tapia and colleagues16 (Figs. 6A & 6B).
Fig. 2
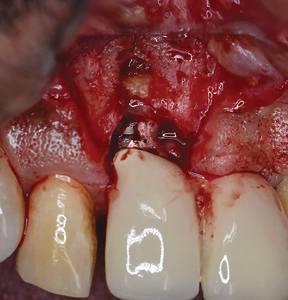
Fig. 3
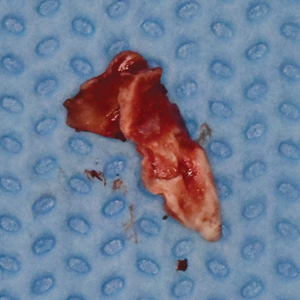
Fig. 4
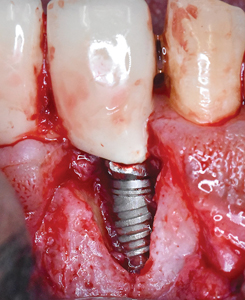
Fig. 5

Fig. 6A

Note the “smoother” appearance of the implant surface after titanium brush treatment.
Fig. 6B

Note the “smoother” appearance of the implant surface after titanium brush treatment.
The bony defect was grafted with BDX (OCS-B; Keystone Dental) (Figs. 7A & 7B) and then covered with ddACM (BioXclude; Snoasis Medical). (Figs. 8A & 8B) The ddACM was adapted intimately to the site and extended at least 2 mm beyond the margins of the newly grafted area, effectively sealing the graft from surrounding tissues. The rounded edge of a periosteal elevator (P24G; Hu-Friedy) that was wetted with saline was used to further adapt the membrane more definitively. The buccal flap was then replaced and closed in a tension-free manner with a sling suture using a 5-0 monocryl suture and the vertical releasing incisions were closed with interrupted, 5-0 chromic gut sutures (Ethicon; Johnson and Johnson). (Fig. 9)
Fig 7A
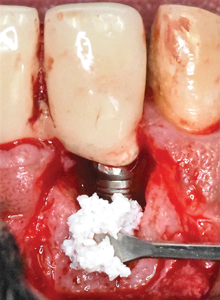
Fig. 7B

Fig. 8A

Fig. 8B

Fig. 9

Post-surgically, the patient was prescribed systemic antibiotics (amoxicillin 500 mg three times daily for 1 week) and a non-steroidal anti-inflammatory analgesic (ketorolac 10 mg, three times daily as needed for pain for 5 days). The patient was also instructed to rinse with warm salt water 4 times daily and to avoid brushing around the implant and immediately adjacent teeth for 2 weeks. The patient was seen at 1- and 2 weeks post-operatively for supra-gingival prophylaxis prior to resumption of brushing and flossing. Sutures were removed after 2 weeks. The patient received periodontal maintenance care every 3 months at his referring dentist’s office. The patient was seen for clinical and radiographic assessment of the surgically treated area at 6 months and 2-years post-operatively.
Clinical Outcomes
Post-operative healing was uneventful and the patient was highly compliant with oral hygiene and homecare. Clinical appearance at 6 months and 2 years showed healthy peri-implant soft tissues (Figs. 10A & 10B) and probing depths along the buccal aspect were measuring 3 mm at the mesio-buccal, mid-buccal, and disto-buccal surfaces with no bleeding on probing or suppuration. In this regard, the patient did not have any pain or tenderness on palpation of the buccal tissues. The corresponding peri-apical radiographs showed complete bony defect fill at 6 months which was sustained over 2 years. (Figs. 11A & 11B)
Fig. 10A

Fig. 10B

Fig. 11A

Fig. 11B

Discussion
Numerous systematic reviews and meta- analyses have evaluated the efficacy of regenerative surgical therapy for the treatment of peri-implantitis.17,18 While regenerative procedures employing GBR using particulate bone in combination with barrier membranes might be more effective, the outcomes of peri-implant regenerative procedures have also been shown to have significant variation and consequently, the predictability and long-term stability of these procedures is still a matter of controversy.
While re-osseointegration of contaminated implant surfaces is possible with the use of regenerative therapies,19 it largely depends on our ability to decontaminate the implant surface. Irrespective of the therapeutic approach, the primary objective in the treatment of peri-implantitis is to remove the biofilm from the implant surface to promote proper healing.20 Furthermore, identification and removal of the principle etiologic factors is also paramount in obtaining a successful outcome. In the case described, the etiology is likely attributable to the presence of retained cement which has been shown to be a risk indicator for the development of peri-implant diseases.21
The use of a titanium brush in this case almost certainly contributed to the observed successful outcome. In vitro investigations have shown that the use of titanium brushes do not significantly alter the microsurface topography of implant surfaces.22 Furthermore, a recent study16 which evaluated the adjunctive effect of a titanium brush for implant surface decontamination during regenerative surgery therapies for peri-implantitis demonstrated a clinically and statistically significant reduction of peri-implant probing depths at 12 months post-operatively. The findings of the case presented in this report are consistent with the results from the aforementioned investigation.
The morphological features of osseous defects surrounding an implant with peri-implantitis can also profoundly influence the outcome of regenerative therapy.23 Schwarz and colleagues have demonstrated that circumferential peri-implant defects are the most prevalent (~55%)24 but also generally most conducive to achieving greater probing depth reduction and clinical attachment level (CAL) gains at 1 year compared to other defect morphologies. Serino and colleagues25 demonstrated that more than one-third of peri-implant defects exhibit a semi-circumferential bone loss pattern with buccal bone dehiscence which is similar to the peri-implant defect described in this case- an advanced Class 1b23 (> 6 mm/>50% of implant length with 2-3 residual bone walls). These defects have been shown to be least favorable for CAL gain.26
In this case report, complete defect resolution and substantial CAL gain (~ 5-7 mm) of an advanced Class 1b osseous defect have been achieved employing a regenerative approach with BDX and ddACM. Healthy and stable peri-implant soft tissues were obtained and maintained successfully over the 2-year follow-up period. These improvements are typically associated with increased survival of implants and decreased risk for recurrence of peri-implantitis.27
While thorough debridement of the defect and meticulous implant surface decontamination with the titanium brush were undoubtedly important for the successful result shown, the choice of barrier membrane is also a critical factor given the severity of the bony defect. For these reasons, the decision was made to use ddACM for its unique physical and biological properties14 thus making it an attractive alternative to the traditionally used collagen-based membranes. To the author’s knowledge, there is only one other case reported in the literature involving the use of ddACM which demonstrated substantial bone fill of the original peri-implant bony defect; in the aforementioned report, the authors used freeze-dried bone allograft and re-entry was done at 1 year.28
ddACM comes as a thin, dehydrated sheet which, upon hydration with saline or contact with oral fluids, becomes pliable and can be intimately adapted to the grafted site. This mechanical exclusion of undesirable soft tissues from growing into the grafted defect while providing adequate space are key biologic requirements for successful bone regeneration.29 The ddACM can also be folded onto itself without additional trimming as is required for conventional resorbable membranes.10 Lastly, the cost of ddACM is comparable to other commercially available conventional resorbable and non-resorbable barrier membranes. In fact, use of ddACM may potentially be more economical given its favorable biologic properties thereby requiring less post-operative management even in the face of complications such as premature membrane exposure.
Conclusion
This case report has demonstrated a novel approach for surgical regenerative treatment of peri-implantitis using BDX and ddACM on a decontaminated implant surface. Re-establishment of peri-implant soft tissue health along with sustained bone fill can be accomplished (at least over a two year period). Within the limitations of a case report where re-entry and histological sampling was not done, one cannot conclusively say that re-osseointegration of the implant or bone regeneration has occurred. Furthermore, it is difficult to quantify the influence of any one component of the surgical technique described here on the healing process and outcome. However, the successful outcome obtained by the technique described in this case report exemplifies the notion that ddACM has potential to be used as a barrier membrane in regenerative therapies for peri-implantitis and is consistent with the author’s experiences with this membrane for other types of surgery where there appear to be substantial and positive effects on healing.30 Further research involving larger case series, controlled clinical trials, and histological analysis using ddACM for this indication will be required to validate more reliably the predictability of the technique described above and to evaluate long-term clinical and radiographic stability of the results obtained.
Oral Health welcomes this original article.
References
- Schwarz F, Derks J, Monje A, Wang HL. Peri-implantitis. J Periodontol 2018;89(Suppl 1):S267-s290.
- Atieh MA, Alsabeeha NHM, Faggion CM, Duncan WJ. The frequency of peri-implant diseases: A systematic review and meta-analysis. J Periodontol 2012;84:1586-1598.
- Lee CT, Huang YW, Zhu L, Weltman R. Prevalences of peri-implantitis and peri-implant mucositis: Systematic review and meta-analysis. J Dent 2017;62:1-12.
- Heitz-Mayfield L, Mombelli A. The therapy of peri-implantitis: A systematic review. Int J Oral Maxillofac Implants 2014;29(Suppl): 325-345.
- Javed F, Alghamdi AS, Ahmed A, Mikami T, Ahmed HB, Tenenbaum HC. Clinical efficacy of antibiotics in the treatment of peri-implantitis. Int Dent J 2013;63:169-176.
- Renvert S, Hirooka H, Polyzois I, Kelekis-Cholakis A, Wang HL. Diagnosis and non-surgical treatment of peri-implant diseases and maintenance care of patients with dental implants – Consensus report of working group 3. Int Dent J 2019;69(Suppl 2):12-17.
- Claffey N, Clarke E, Polyzois I, Renvert S. Surgical treatment of peri-implantitis. J Clin Periodontol. 2008 Sep;35(8 Suppl):316-32.
- Roos-Jansåker AM, Lindahl C, Persson GR, Renvert S. Long-term stability of surgical bone regenerative procedures of peri-implantitis lesions in a prospective case-control study over 3 years. J Clin Periodontol 2011;38:590-597.
- Schwarz F, Hegewald A, John G, Sahm N, Becker J. Four-year follow-up of combined surgical therapy of advanced peri-implantitis evaluating two methods of surface decontamination. J Clin Periodontol. 2013 Oct;40(10):962-7
- Cullum D, Lucas M. Minimally invasive extraction site management with dehydrated amnion/chorion membrane (dHACM): Open-socket grafting. Compend Contin Educ Dent 2019;40:178–183.
- Temraz A, Ghallab NA, Hamdy R, El-Dahab OA. Clinical and radiographic evaluation of amnion chorion membrane and demineralized bone matrix putty allograft for management of periodontal intrabony defects: a randomized clinical trial. Cell Tissue Bank 2019;20:117-128.
- Holtzclaw D. Maxillary sinus membrane repair with amnion–chorion barriers: A retrospective case ceries. J Periodontol 2015;86:936-940.
- Warning JC, McCracken SA, Morris JM. A balancing act: Mechanisms by which the fetus avoids rejection by the maternal immune system. Reproduction 2011;141:715-724.
- Koob TJ, Rennert R, Zabek N, Massee M, Lim JJ, Temenoff JS, Li WW, Gurtner G. Biological properties of dehydrated human amnion/chorion composite graft: implications for chronic wound healing. Int Wound J. 2013;10:493-500.
- Ashraf H, Font K, Powell C, Schurr M. Antimicrobial activity of an amnion-chorion membrane to oral microbes. Int J Dent. 2019 Jul 11;2019:1269534.
- de Tapia B, Valles C, Ribeiro-Amaral T, Mor C, Herrera D, Sanz M, Nart J. The adjunctive effect of a titanium brush in implant surface decontamination at peri-implantitis surgical regenerative interventions: A randomized controlled clinical trial. J Clin Periodontol 2019;46:586-596
- Sahrmann P, Attin T, Schmidlin PR. Regenerative treatment of peri-implantitis using bone substitutes and membrane: A systematic review. Clinical Implant Dent Rel Res 2011;13:46-57.
- Daugela P, Cicciù M, Saulacic N. Surgical regenerative treatments for peri-implantitis: Meta-analysis of recent findings in a systematic literature review. J Oral Maxillofac Res 2016;7:e15.
- Madi M, Htet M, Zakaria O, Alagl A, Kasugai S. Re-osseointegration of dental implants after periimplantitis treatments: A systematic review. Implant Dent 2018;27:101-110.
- Monje A, Pons R, Roccuzzo A, Salvi GE, Nart J. Reconstructive therapy for the management of peri-implantitis via submerged guided bone regeneration: A prospective case series. Clin Implant Dent Relat Res 2020;22:342-350.
- Staubli N, Walter C, Schmidt JC, Weiger R, Zitzmann NU. Excess cement and the risk of peri-implant disease–a systematic review. Clin Oral Implants Res 2017;28:1278-1290.
- Park JB, Jeon Y, Ko Y. Effects of titanium brush on machined and sand-blasted/acid-etched titanium disc using confocal microscopy and contact profilometry. Clin Oral Implants Res 2015;26:130-136.
- Monje A, Pons R, Insua A, Nart J, Wang HL, Schwarz F. Morphology and severity of peri-implantitis bone defects. Clin Implant Dent Relat Res 2019;21:635-643.
- Schwarz F, Herten M, Sager M, Bieling K, Sculean A, Becker J. Comparison of naturally occurring and ligature-induced peri-implantitis bone defects in humans and dogs. Clin Oral Implants Res 2007;18:161-170.
- Serino G, Turri A, Lang NP. Probing at implants with peri-implantitis and its relation to clinical peri-implant bone loss. Clin Oral Implants Res 2013;24:91-95.
- Schwarz F, Sahm N, Schwarz K, Becker J. Impact of defect configuration on the clinical outcome following surgical regenerative therapy of peri-implantitis. J Clin Periodontol 2010;37:449-455.
- Carcuac O, Derks J, Abrahamsson I, Wennström JL, Berglundh T. Risk for recurrence of disease following surgical therapy of peri-implantitis–A prospective longitudinal study. Clin Oral Implants Res 2020;31:1072-1077.
- Froum S, Rosen P. Reentry evaluation following treatment of peri-implantitis with a regenerative approach. Int J Periodontics Restorative Dent 2014;34:47-59.
- Wang HL, Boyapati L. “PASS” principles for predictable bone regeneration. Implant Dent 2006;15:8-17.
- Bhide VM, Tenenbaum HC. Treatment of gingival genestration with a double pedicle flap approach and dehydrated amnion-chorion membrane: A case report. Clin Adv Periodontics 2020;10: 200-203.
About the Author
 Dr. Vinay Bhide is a board-certified periodontist who provides the full scope of surgical periodontal and dental implant therapy. Dr. Bhide has a special interest in esthetic and reconstructive periodontics. In addition to private practice, Dr. Bhide is a clinical instructor in the Department of Periodontics at the University of Toronto where he is involved in didactic and clinical teaching of periodontics at both undergraduate and post-graduate levels. He is also an Adjunct Assistant Professor in the Department of Periodontics at UPenn. Dr. Bhide has lectured nationally and internationally and published on various topics related to periodontics and implantology. He can be reached at vinay.bhide@utoronto.ca Private practice, Aurora, Ontario.
Dr. Vinay Bhide is a board-certified periodontist who provides the full scope of surgical periodontal and dental implant therapy. Dr. Bhide has a special interest in esthetic and reconstructive periodontics. In addition to private practice, Dr. Bhide is a clinical instructor in the Department of Periodontics at the University of Toronto where he is involved in didactic and clinical teaching of periodontics at both undergraduate and post-graduate levels. He is also an Adjunct Assistant Professor in the Department of Periodontics at UPenn. Dr. Bhide has lectured nationally and internationally and published on various topics related to periodontics and implantology. He can be reached at vinay.bhide@utoronto.ca Private practice, Aurora, Ontario.












