In recent decades, dental implants have emerged as a valuable tooth replacement option, offering favorable esthetic outcomes and long-term stability. Despite their high success rates, implants are subject to biological complications that compromise peri-implant tissues,1 peri-implant mucositis and peri-implantitis being two of the most common inflammatory conditions.2 Current epidemiological studies highlight the high prevalence of peri-implant mucositis, one that affects many dental implant patients. As many as 90% of peri-implant tissues have some form of inflammatory response and the prevalence of peri-implantitis ranges 28-51%.3 Peri-implant mucositis is characterized by an inflammatory response confined to the soft tissues surrounding an implant, with no radiographic evidence of bone loss.4
Clinically, peri-implant mucositis is characterized by redness, swelling, and bleeding on probing, but is reversible if diagnosed early and managed with professional debridement and improved oral hygiene practices.5,6 Additionally, the prophylactic use of photobiomodulation therapy (PBM), the application of low levels of laser energy, has the potential to help reduce this inflammatory response.7 Untreated peri-implant mucositis can progress to peri-implantitis, a more severe condition involving progressive bone loss around the implant, and potential failure of the implant-prosthesis. The diagnosis and management of peri-implant mucositis is all too often ignored by both the patient and oral health clinicians, allowing the condition to progress.8
Peri-implantitis affects the surrounding soft tissues and also undermines the alveolar bone, compromising the implant’s structural integrity and long-term prognosis. Peri-implantitis is staged based on tissue destruction severity and clinical parameters,2 including bleeding on probing, suppuration, increased probing depths, and radiographic evidence of crater-like bone loss.9,10 Treatment approaches for peri-implantitis vary considerably, from nonsurgical debridement and adjunctive antimicrobial therapies to complex surgical interventions such as guided bone regeneration.6
Progression of peri-implant mucositis to peri-implantitis
The transition from mucosal inflammation to peri-implant bone loss is of great clinical concern. Various longitudinal studies suggest that approximately 10-20% of implants with untreated peri-implant mucositis will eventually manifest signs of peri-implantitis.11 Although reported disease progression rates vary, it is clear that early detection and appropriate intervention are paramount for prevention, minimizing bone loss and implant failure.
Read: Current management strategies for peri-implant diseases
Of late, laser therapy is increasingly viewed as an adjunct or alternative to conventional debridement techniques.9 Lasers offer several advantages, including bactericidal effects, selective ablation of diseased tissues, and management of intraoperative bleeding during procedures. Different laser wavelengths (e.g., Er:YAG, Nd:YAG, and various diode lasers) have been explored for their ability to decontaminate implant surfaces without causing thermal damage to the surrounding tissues or altering the implant surface microstructure.10
Implant case selection and risk assessment
A critical yet sometimes underemphasized element in preventing peri-implant diseases is the selection of appropriate implant candidates and meticulous treatment planning. Comprehensive preoperative evaluations improve implant success rates and also minimize the risk of postoperative complications, including peri-implant mucositis and peri-implantitis.12
Patient history and systemic considerations
An essential first step is the detailed medical and dental history to identify systemic conditions that may compromise healing or elevate the risk of infection.1 Patients with poorly controlled diabetes, autoimmune disorders, or a history of head and neck radiation warrant a more cautious approach due to potential immunological challenges and impaired regenerative capacity.8 In addition, periodontal history, smoking, dental habits (bruxism) and other lifestyle factors can significantly impact peri-implant tissue health and should be thoroughly assessed and, if possible, modified prior to implant therapy.13
Periodontal status
A history of periodontal conditions is strongly correlated with an increased risk of implant complications. Residual periodontal pockets, persistent bleeding on probing, and inadequate plaque control are indicators that additional periodontal therapy and patient education may be needed. Before implant placement, all active periodontal disease must be addressed and controlled for an appropriate length of time. Stabilizing the periodontal conditions provides a healthier oral environment that supports long-term implant success.14,15
Local oral conditions and bone quality
The quality and quantity of alveolar bone must be thoroughly evaluated. Cone-beam computed tomography (CBCT) imaging is required to assess bone volume, density, and anatomical structures (e.g., proximity to nerves or the sinus floor).16 Adequate bone height and width are critical for achieving primary stability, a key prerequisite for predictable osseointegration. In cases of insufficient bone volume, advanced procedures like guided bone regeneration (GBR) or sinus augmentation may be necessary before implant placement.10
Occlusal and prosthetic considerations
A balanced occlusal scheme distributes masticatory forces evenly across the arch and around the implant, reducing micromovements and stress concentrations that can disrupt peri-implant bone remodeling.6 Prior to implant placement, the treatment team should determine the appropriate implant position, angulation, and prosthetic design, ensuring that restorative procedures do not preclude adequate oral hygiene and do not impose undue forces on the implant.8
Behavioral and maintenance factors
Long-term success also hinges on the patient’s ability and willingness to perform rigorous home care. Where possible, patients should be counseled on the importance of meticulous daily plaque control, regular use of a night time occlusal appliance, regular professional preventive care, and smoking cessation.13 An individualized recall schedule at a minimum of every 3 months, with intervals determined by the patient’s risk profile, can detect early peri-implant tissue changes and lead to timely intervention.11
Reducing the burden of peri-implant diseases
By addressing these multifactorial considerations before implant placement, clinicians can significantly reduce the likelihood of peri-implant mucositis and peri-implantitis in the long term. This patient-centered, preemptive approach aligns with evolving trends in implant dentistry, which emphasize not only surgical proficiency but also detailed risk stratification, prevention, and maintenance. Consequently, case selection is no longer confined to evaluating purely local anatomical feasibility; it extends to comprehensively managing systemic conditions, behavioral habits, biomechanical forces, and existing oral diseases that collectively affect peri-implant tissue health.
Multifactorial etiology of peri-implant diseases
Both peri-implant mucositis and peri-implantitis arise from multiple interacting factors including microbial biofilms, host immune responses, and environmental or iatrogenic influences. Plaque biofilm is typically identified as the primary etiological factor driving the onset of inflammation, akin to the pathogenic mechanisms seen in periodontal disease around natural teeth. However, the progression toward peri-implantitis is often expedited by additional factors, such as:
- History of Periodontitis: Patients with untreated or poorly controlled periodontal disease are more prone to developing peri-implantitis.8
- Systemic Conditions: Conditions such as diabetes mellitus can exacerbate the inflammatory response, impair wound healing, and increase susceptibility to implant complications.8
- Smoking: Tobacco use is a significant risk factor for peri-implant bone loss.13
- Excessive Occlusal Loading: Poorly designed prostheses or occlusal overload can predispose implants to micromovements that disrupt peri-implant homeostasis.6
- Improper Implant Positioning or Design: Suboptimal implant placement, poorly designed, inappropriately contoured and ill-fitting restorations can create niches for biofilm accumulation, challenging adequate cleaning and fostering inflammation.8
Given this multifactorial etiology, a single therapeutic modality is seldom sufficient to address every risk element. Typically, a comprehensive strategy—involving patient education, meticulous plaque control, occlusal assessment, and tailored follow-up intervals—is critical for preventing disease progression.1
Evolution of treatment strategies
Historically, peri-implant disease management centered on mechanical debridement of contaminated implant surfaces, supplemented by antiseptic irrigation and/or adjunctive antimicrobial agents. Scaling and debridement (adapted from periodontal therapy) were extrapolated to the implant environment, with instruments designed to minimize implant surface damage.5 Early reliance on chlorhexidine mouthrinses and local-delivery antibiotics was beneficial in mild mucosal inflammation, these measures proved insufficient for advanced peri-implant bone loss.
As implantology and periodontology advanced, peri-implantitis treatment protocols have diversified. Current management strategies can be broadly categorized into preventive, nonsurgical, and surgical interventions:
- Preemptive Preventive Maintenance: Low-level laser energy (known as photobiomodulation therapy or PBM) and routine implant maintenance therapy help manage inflammation. (Fig. 1)
- Nonsurgical Therapy: Mechanical debridement with specialized ultrasonics or titanium curettes, in conjunction with locally-delivered antibiotics, photodynamic therapy, and/or laser decontamination.16
- Surgical Therapy: Open-flap debridement may be performed for advanced peri-implantitis to gain access to the defect for thorough decontamination. Depending on defect morphology, bone regeneration (bone grafting and guided tissue regeneration) or resective approaches are employed.15
Fig. 1
Given the complexity and multifactorial nature of peri-implant diseases, a collaborative, patient-centered model of care is essential. Successful treatment protocols require:
- Stringent Biofilm Control: Reinforced home care routines, regular professional maintenance, and targeted anti-inflammatory and antimicrobial strategies.
- Risk Factor Modification: Smoking cessation, systemic conditions control(e.g., diabetes), and evaluation of occlusal forces.
- Individualized Treatment Protocols: Tailoring nonsurgical, surgical, and laser procedures to each patient’s specific needs and disease severity.
- Long-Term Monitoring: Regular follow-up visits including detailed clinical and radiographic evaluations to detect early signs of relapse or progression.9
Laser-based therapies
The prevention and management of peri-implant conditions requires a multimodal approach. Lasers have emerged as an adjunctive/ alternative treatment modality for implant maintenance. Various laser wavelengths, such as Er:YAG, Nd:YAG, CO2, and various diode lasers, have been investigated for their capability to decontaminate, manage inflammation, and selectively ablate diseased tissue, creating an ideal environment for tissue regeneration. Properly designed and implemented laser-based therapies reduce surgical complications by decontaminating the implant surface and surrounding biological structures without damaging the implant surface, creating a more ideal healing environment.
The laser wavelength determines its absorption into the target. Nd:YAG 1,064nm laser energy (commonly used for welding metal partial dental frameworks) is also highly absorbed by titanium and can damage an implant even at low energy levels (Fig. 2). Diode wavelengths are absorbed at different rates into the implant fixture and can cause terminal thermal damage to the surrounding structures.17,18
Fig. 2

Laser peri-implantitis management research has focused on decontamination, implant surface changes, or thermal effects separately rather than their combined impact. However, it is the cumulative effect that determines the outcome.
The laser- target interaction is determined by the target’s composition; in peri-implantitis the targets include implant, bone, soft tissue, granulomatous tissue, blood, and various pathogens. Furthermore, the parameters of the laser energy, the wavelength, the amount of energy, and how it is delivered all play a crucial role.
Preventive maintenance
Much like other health-related fields, the primary objective in implantology is to prevent pathology. As previously noted, up to 90% of implants exhibit inflammatory components. Photobiomodulation (PBM) therapy reduces inflammation and promotes the healing of both soft and hard oral tissues.19 PBM therapy on both facial and lingual aspects should be a part every implant preventive maintenance session. PBM therapy parameters are device-dependent, emphasizing the need for device-specific training and education.
Nonsurgical therapy
Nonsurgical therapies are an extension of preventive peri-implant mucositis maintenance. While often effective, non-surgical management of peri-implantitis is less predictable due to limited access to the subgingival implant surface. Nonsurgical therapy includes plaque control, prosthesis design and/or modification to facilitate hygiene, and mechanical debridement.
The peri-implant pocket biofilm must be addressed in nonsurgical therapy. Photon-initiated photoacoustic streaming (shock wave enhanced emission photoacoustic streaming or SWEEPS) is the process of using very short pulses of 50 microseconds or less of laser energy generated by an Er:YAG 2,940nm laser to create bubbles in a fluid irrigant. The bubble expansions and contractions within the irrigant produce shock waves that break down and remove the biofilm in both the periodontal and peri-implant pocket. The SWEEPS procedure is either a series of single pulses or a more aggressive dual pulse technique. In the dual pulse mode, a second ultra short pulse of laser energy is emitted before the primary bubble has contracted, providing a stronger shock wave to break up and remove the biofilm and flush out the pocket. SWEEPS is a relatively short, non-invasive and beneficial procedure essential to nonsurgical therapy.
Plaque control involves multimodal mechanical debridement utilizing curettes, prophy cups, air-polishers, ultrasonic scalers, and SWEEPS. Antiseptic irrigation and rinses (0.12% Chlorhexidine, weak Sodium Hypochlorite (NaOCl)=Dakin’s solution) can be used. Finally, either systemic or local delivery antibiotics may be prescribed.
Surgical therapy
Studies have shown that Er:YAG 2,940nm and CO2 10,600nm lasers have the least thermal effect on titanium implant surfaces. Furthermore, the Er:YAG laser is effective in decontaminating and recontouring bone without creating a smear layer. The Er:YAG 2,940nm laser, at 100 milliJoules (mJ) with water irrigation generated a temperature increase of less than 2°C over 60 seconds. Yet it was effective in decontaminating the implant and surrounding tissue without damaging the implant surface. The same Er:YAG laser at 300mJ without water irrigation has the potential to significantly increase the implant temperature and damage its surface.20 Laser science is crucial, and science-based laser training is mandatory.
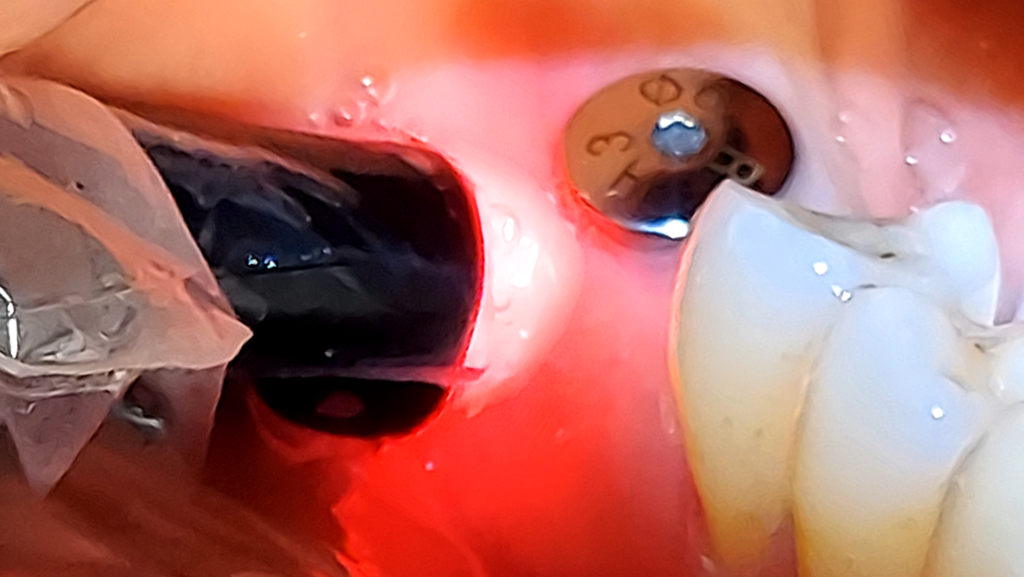
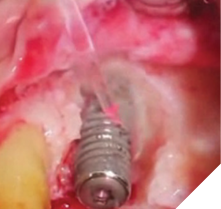
Surgical peri-implantitis treatment involves raising a flap for proper visualization, assessment, and access. An Er:YAG 2,940nm laser with the highest degree of absorption in water, allows for extensive water irrigation during the peri-implantitis procedure. The LightWalker ATS Er:YAG laser 2,940nm (Fotona Dental Lasers, National Dental Innovations, Barrie ON), at appropriate settings, removes granulomatous and diseased soft tissue from the implant surface and surrounding areas without heating or damaging the implant surface (Fig. 3). The laser energy of the 2,940nm effectively decontaminates the implant surface and surrounding bone structures without generating a smear layer on the osseous tissue. The absence of a smear layer on the bone facilitates an optimal environment for the blood-borne growth factors to nourish the bone graft, enhancing the regeneration of hard tissue that stabilizes and supports the implant.
Fig. 3
Conclusion
Peri-implant conditions are increasing exponentially, requiring the adoption of efficient, proactive treatments. Effective management begins with thorough pre-treatment planning, appropriate placement, and restoration. Once the implant is placed, it is crucial to put preventive measures in place and, when necessary, apply early intervention strategies.
While emerging research continues to guide clinicians towards optimal parameters and applications, it is important to act promptly to prevent existing conditions from deteriorating. The prophylactic use of PBM can be a simple first step to help control the ever-present inflammatory process. The Er:YAG 2,940nm laser is an effective method for both non-surgical and surgical peri-implantitis therapy. Practitioners are optimistic about the integration of lasers into implant therapy. However, comprehensive education and device-specific training are essential for clinicians to understand the unique functionalities and principles of each device. Furthermore, it is essential for clinicians to comprehend the biological characteristics of the tissue, its physiological reactions, and its interactions with laser energy to achieve optimal results. The goal is to create a stable, cleansable, and maintainable environment for long-term implant health. 
Oral Health welcomes this original article.
References
- Berglundh et al., “Peri-implant diseases and conditions: Consensus report of workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions,” J. Periodontol., vol. 89, pp. S313–S318, 2018.
- S. J. Froum and P. S. Rosen, “A proposed classification for peri-implantitis,” Int. J. Periodontics Restorative Dent., vol. 32, no. 5, p. 533, 2012.
- V. Astolfi et al., “Incidence of peri-implantitis and relationship with different conditions: a retrospective study,” Int. J. Environ. Res. Public. Health, vol. 19, no. 7, p. 4147, 2022.
- N. U. Zitzmann and T. Berglundh, “Definition and prevalence of peri-implant diseases,” J. Clin. Periodontol., vol. 35, pp. 286–291, 2008.
- A. Mombelli, M. Van Oosten, E. Schürch Jr, and N. Lang, “The microbiota associated with successful or failing osseointegrated titanium implants,” Oral Microbiol. Immunol., vol. 2, no. 4, pp. 145–151, 1987.
- L. J. Heitz-Mayfield, I. Needleman, G. E. Salvi, and B. E. Pjetursson, “Consensus statements and clinical recommendations for prevention and management of biologic and technical implant complications.,” Int. J. Oral Maxillofac. Implants, vol. 29, 2014.
- R. S. Saini et al., “Comparative Efficacy of Photobiomodulation on Osseointegration in Dental Implants: A systematic review and meta-analysis,” Photodiagnosis Photodyn. Ther., p. 104256, 2024.
- S. Jepsen et al., “Periodontal manifestations of systemic diseases and developmental and acquired conditions: Consensus report of workgroup 3 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions,” J. Clin. Periodontol., vol. 45, pp. S219–S229, 2018.
- A. Mombelli, “Microbiology and antimicrobial therapy of peri-implantitis,” Periodontol. 2000, vol. 28, no. 1, pp. 177–189, 2002.
- Schwarz, J. Derks, A. Monje, and H.-L. Wang, “Peri-implantitis,” J. Clin. Periodontol., vol. 45, pp. S246–S266, 2018.
- O. C. Koldsland, A. A. Scheie, and A. M. Aass, “Prevalence of peri-implantitis related to severity of the disease with different degrees of bone loss,” J. Periodontol., vol. 81, no. 2, pp. 231–238, 2010.
- D. Buser, L. Sennerby, and H. De Bruyn, “Modern implant dentistry based on osseointegration: 50 years of progress, current trends and open questions,” Periodontol. 2000, vol. 73, no. 1, pp. 7–21, 2017.
- C.-T. Lee, Y.-W. Huang, L. Zhu, and R. Weltman, “Prevalences of peri-implantitis and peri-implant mucositis: systematic review and meta-analysis,” J. Dent., vol. 62, pp. 1–12, 2017.
- S. W. Lee, “Association of Immediate Dental Implant Survival with Periodontal Disease and Periodontal Maintenance Status,” Master’s Thesis, College of Medicine-Mayo Clinic, 2020.
- N. de Campos Kajimoto, Y. de Paiva Buischi, M. Mohamadzadeh, and P. Loomer, “The Oral Microbiome of Peri-Implant Health and Disease: A Narrative Review,” Dent. J., vol. 12, no. 10, p. 299, 2024.
- M. Sanz, I. L. Chapple, and W. G. 4 of the V. E. W. on Periodontology*, “Clinical research on peri-implant diseases: consensus report of W orking G roup 4,” J. Clin. Periodontol., vol. 39, pp. 202–206, 2012.
- C. Leja, A. Geminiani, J. Caton, and G. E. Romanos, “Thermodynamic effects of laser irradiation of implants placed in bone: an in vitro study,” Lasers Med. Sci., vol. 28, pp. 1435–1440, 2013.
- Valente NA, Calascibetta A, Patianna G, Mang T, Hatton M, Andreana S. Thermodynamic Effects of 3 Different Diode Lasers on an Implant-Bone Interface: An Ex-Vivo Study With Review of the Literature. J Oral Implantol. 2017 Apr;43(2):94-99. doi: 10.1563/aaid-joi-D-16-00188. Epub 2016 Dec 21. PMID: 28001482.
- Gholami L, Asefi S, Hooshyarfard A, Sculean A, Romanos GE, Aoki A, Fekrazad R.
- Photobiomodulation in Periodontology and Implant Dentistry: Part 1 & 2
- Photobiomodulation Photomed Laser Surg. 2019 Dec;37(12):739-783
- Geminiani A, Caton JG, Romanos GE. Temperature increase during CO(2) and Er:YAG irradiation on implant surfaces. Implant Dent. 2011 Oct;20(5):379-82. doi: 10.1097/ID.0b013e3182310d57. PMID: 21881518.
About the authors

Dr. Ghaffarinia, periodontal resident at Oregon Health & Science University, earned her DMD from Midwestern University College of Dental Medicine and now advances expertise in periodontics and implantology.

Dr. Cianciola is Clinical Assistant Professor of Periodontology at Midwestern University; former 14-year managing partner in Rochester, NY, expert in cosmetic and implant surgery, Misch-trained, Faculty for Resnik Implant Institute.

Dr. Benjamin is in private practice in upstate NY and has faculty appointments at several universities. He is the Chairman of the ADA Standards Committee Working Group on Dental Lasers, a Past President of the Academy of Laser Dentistry and is a Faculty Expert with the Laser & Health Academy.