Summary
This case report provides a review of the use of the intramuscular (IM) injection of glycopyrrolate bromide as an antisialagogue. In this case, copious saliva interfered with the ability to obtain an accurate impression of the tooth preparation. Glycopyrrolate was delivered intramuscularly to the deltoid muscle. Drying was adequate, and a successful impression was obtained.
Patient History
A 46-year-old male presented to the University of Toronto Faculty of Dentistry clinic for fabrication of a fixed prosthesis from tooth 44 to tooth 46. His medical history was non-contributory, other than a smoking history of 12.5 pack years. Following tooth preparation, a final impression using polyvinyl siloxane was attempted. Despite otherwise optimal conditions, multiple attempts failed to adequately capture the bridge abutments due to imperfect margins caused by the presence of saliva. Given repeated failed attempts, it was decided that an intervention was required to control excess salivary flow.
What is the problem?
Hypersalivation often occurs in response to stimulation but can also be a resting condition. Psychoactive drugs such as classical and novel antipsychotic drugs can also cause hypersalivation. For example, clozapine, which is prescribed for patients with schizophrenia, increases salivation in approximately 50% of patients.1 This patient was not taking any medications that would result in excess salivary production. His daily habit of smoking would be more likely to cause dry mouth, as smoking is known to reduce salivary excretion and to change the quality of saliva.2,3 The patient did not complain of excess salivation or drooling. Therefore, it was assumed that the high salivary production was likely due to an above-average resting salivary rate.
What are the potential solutions?
Salivation is controlled by dual innervation from both the sympathetic (noradrenergic) and parasympathetic (cholinergic) autonomic nervous systems. Autonomic control is mediated by actions at neuroreceptors and synapses. Salivation and hypersalivation results from: the stimulation of muscarinic cholinoceptors or dopamine D2 receptors; the blockade of alpha-2-adrenoreceptors; and/or the depletion of noradrenaline from central stores. Conversely, blockade of the muscarinic cholinoceptors or the alpha-1-adrenoceptors, inhibition of noradrenaline update, and/or stimulation of alpha-2-adrenoreceptors all lead to hyposalivation.
In the dental office, antimuscarinic agents may be used to suppress salivary production. Common antisialagogues in this category include atropine sulfate and glycopyrrolate bromide.3 While atropine does have a drying effect, this action has been reported as unreliable. Its further disadvantages include a short duration of action and more potent cardiovascular effects.3 Glycopyrrolate is five to six times more potent than atropine as an antisialagogue, and has selective and prolonged effects on salivary secretion.4,5 High doses of glycopyrrolate do not cause tachycardia or significant increases in heart rate. When compared to atropine, glycopyrrolate has fewer cardiovascular, ocular and central nervous system effects, mainly due to its high polarity rendering it unable to cross the blood-brain barrier.4,5 The effect of glycopyrrolate on salivary production is dose-related and the peak effect is attained in one hour.6 Despite these desirable qualities, glycopyrrolate use must still be utilized with care to minimize undesirable side effects (Table 1).
Table 1
Clinical Protocol and Dosage Calculation
The patient was advised to arrive 45 minutes prior to the appointment for check-in and delivery of the IM injection. He was instructed to bring a water bottle for sipping post-operatively to hydrate his mouth, he was told to expect to experience a dry mouth for approximately six hours after his appointment. The injection was provided 30 minutes before the procedure to match its peak action with the timing of the impression, which we expected to occur in 60 minutes.
The recommended standard adult dose of glycopyrrolate (IM) injection for its antisialagogue properties is 0.004 mg/kg. Weight-based dosing at 0.005-0.01 mg/kg can also be used if basing the dose on lean body mass rather than actual body weight in an obese patient. The maximum dose is 0.2-0.3 mg.6
This patient was 74.8 kg with a normal BMI. Our weight-based calculation was as follows:
74.8 kg x 0.004mg/kg = 0.3 mg
To deliver a 0.3 mg dose of the 0.2 mg/mL formulation, 1.5 mL was injected in the right deltoid muscle. (Figures 1 & 2) After 30 minutes, the clinical observations included mild reduction of saliva on the floor of mouth and at the buccal mucosa. With the control of salivary production, it was possible to successfully take a polyvinyl siloxane impression of the bridge abutments without any voids in the impression material. The final impression has been accepted for the fabrication of the bridge. The patient reported overall comfort with the procedure and did not report experiencing a dry mouth afterwards. In the dental practice, glycopyrrolate IM injections are a safe and effective way to control hypersalivation, with minimal cardiovascular, ocular and central nervous system side effects.
Fig. 1A

Fig. 1B

Fig. 1C

Fig. 1D

Fig. 2A

Fig. 2B
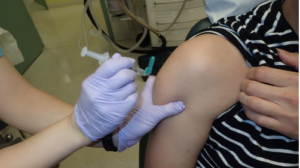
Fig. 2C

Discussion and Drug Information
Administering a drug should be done with a clear understanding of the drug’s pharmacology and the patient’s medical history. The drug should then be delivered with caution.
Some of the appreciable side effects of glycopyrrolate IM injection are constipation, nausea, vomiting, insomnia, decreased sweating, flushing or fever, headache, painful urination, tachycardia, and confusion.6
Be aware of common drug interactions with glycopyrrolate, including atenolol, cannabinoid-containing products, ipratropium, metformin, nitroglycerin, opioid agonists, and thiazide-like diuretics.6 (Table 1)
Contraindications to the use of glycopyrrolate include previous hypersensitivity reactions to glycopyrrolate or any components of its formulation, and medical conditions that preclude the use of anticholinergic medication, such as severe ulcerative colitis, toxic megacolon complicating ulcerative colitis, paralytic ileus, obstructive disease of the gastrointestinal tract, intestinal atony in elderly or debilitated patients, unstable cardiovascular status in acute hemorrhage, glaucoma, obstructive uropathy, and myasthenia gravis.6
Conclusion
Consider administering an antisialagogue as part of your dental protocol when saliva is contaminating the operative field (e.g. prior to taking an impression, when there have been multiple failed impressions). The IM administration of medications is relatively simple, and will save the practitioner and patient repeated attempts at taking impressions, as well as the associated costs (i.e. chair time and materials). Glycopyrrolate bromide is effective at decreasing the quantity of saliva with minimal appreciable side effects in the healthy patient.
Oral Health welcomes this original article.
References
- Davydov L, Botts SR. Clozapine-induced hypersalivation. Annals of Pharmacother. 2000 May;34(5):662-665. doi.org/10.1345/aph.19259.
- Dyasanoor S, Saddu SC. Association of xerostomia and assessment of salivary flow using modified Schirmer Test among smokers and healthy individuals: A preliminary study. J Clin Diagn Res. 2014 Jan;8(1):211-213. doi: 10.7860/JCDR/2014/6650.3846.
- Kazen DH, Dille JM. An evaluation of atropine as an antisialagogue in dentistry. Oral Surg, Oral Med, Oral Pathol. 1963 Aug;16(8):919-925. doi.org/10.1016/0030-4220(63)90191-9.
- Mirakhur RK, Dundee JW. Comparison of the effects of atropine and glycopyrrolate on various end-organs. J R Soc Med. 1980 Oct;73(10):727-730. PMID: 7241426.
- Kongsrud F, Sponheim S. A comparison of atropine and glycopyrrolate in anaesthetic practice. Acta Anaesthesiol Scand. 1982 Dec;26(6):620-625. PMID: 7158272.
- Glycopyrrolate (systemic). In: Lexi-drugs online [database on the Internet]. Hudson (OH): Lexicomp Inc.; [updated 2020 Aug 11; cited 2020 Nov 15]. Available from: http://online.lexi.com.myaccess.library.utoronto.ca/lco/action/doc/retrieve/docid/patch_f/5911775?cesid=8aFJr3WsHxs&searchUrl=%2Flco%2Faction%2Fsearch%3Fq%3Dglycopyryrolate%26t%3Dname%26va%3Dglycopyryrolate#rfs
About the Authors
 Dr. Amanda Chiu obtained her DDS from University of Toronto, completed her general practice residency at Sunnybrook Health Sciences Center and is now pursuing her residency training in dental anaesthesia at the University of Toronto. She can be reached at amandavincci.chiu@mail.utoronto.ca.
Dr. Amanda Chiu obtained her DDS from University of Toronto, completed her general practice residency at Sunnybrook Health Sciences Center and is now pursuing her residency training in dental anaesthesia at the University of Toronto. She can be reached at amandavincci.chiu@mail.utoronto.ca.
 Dr. Carilynne Yarascavitch is a Board-Certified Specialist in Dental Anaesthesiology. She is an Assistant Professor at the Faculty of Dentistry, University of Toronto and Active Staff in the Department of Dentistry at Sunnybrook Health Sciences Centre in Toronto, Ontario, where she provides dentistry and anesthesia services for medically compromised and special needs patients. She can be reached at c.yarascavitch@dentistry.utoronto.ca.
Dr. Carilynne Yarascavitch is a Board-Certified Specialist in Dental Anaesthesiology. She is an Assistant Professor at the Faculty of Dentistry, University of Toronto and Active Staff in the Department of Dentistry at Sunnybrook Health Sciences Centre in Toronto, Ontario, where she provides dentistry and anesthesia services for medically compromised and special needs patients. She can be reached at c.yarascavitch@dentistry.utoronto.ca.
Click here to see more articles from the February 2021 issue of Oral Health!













