Introduction:
Desmoid tumor (DT), desmoid fibromatosis (DF) or aggressive fibromatosis, is a rare, benign, but locally aggressive, soft tissue tumor.1 The most common sites are the abdominal wall, mesentery, and chest wall.2 Approximately 7-15% of DTs occur in the head and neck with the most common subsite being the supraclavicular fossa. It appears, particularly in the head and neck subsites, to be a disease of children and young adults with a median age of 30 years, although it has been reported from newborns to the elderly.3 In most series it is more common in females than males.2,3 DTs are a fibrous neoplasm that can arise in connective tissues like skeletal muscle, fascia, and periosteum and although locally destructive, DTs cannot metastasize.4 They are not encapsulated and have the ability to infiltrate along fascial planes and invade neurovascular structures and bone. This pattern of infiltration and local destruction may mimic malignant entities complicating the diagnostic process. Because of its aggressive behavior and locally destructive pattern, it can cause functional impairment and can be life threatening on rare occasions. This aggressive behavior also makes complete surgical resection challenging with only 11% of children and 38-55% of adults having clear surgical margins.3,5
There have only been a small number of cases of DTs reported in the head and neck region with most reports being in children. This is a case report of a young adult female with a large and aggressive masticator space DT treated with surgical resection. The case represented both a challenge in the diagnosis and treatment. Given the scarcity of information on this tumor, its aggressive nature, and the often young age of patients that are affected by it, there are controversies as to the best approach to treatment: balancing the risk of disease recurrence with patient function and the long-term morbidity of the various treatment options.
Case Report:
A 24-year-old healthy female patient was referred to the Department of Oral and Maxillofacial Surgery at the Montreal General Hospital for progressive trismus and pain three months following removal of her impacted tooth #48. The patient initially attributed her symptoms to her recent extraction and delayed presentation to her original surgeon until three months following the extraction.
On examination, she was found to have a maximal interincisal opening of 10 mm, a large buccal space submucosal mass with extension into the masticator space, right lingual nerve paresthesia, and a palpable, mobile, right level 1B enlarged lymph node. On retrospective review of the panorex taken prior to her wisdom tooth extraction, a subtle radiolucency of the right mandibular ramus could be appreciated. (Fig. 1) Her initial investigations included a computed tomography (CT) scan of her face and neck, magnetic resonance imaging (MRI) of her face, and an incisional biopsy. The imaging was significant for a 6.2 x 6.1 x 3.0 cm mass centered in the right masticator space invading the mandibular ramus laterally with involvement of the masseter muscle, the deep lobe of the parotid posterolaterally, the posterior maxilla and pterygoid plates posteromedially, while abutting the internal carotid artery posteriorly and the skull base superiorly. (Fig. 2) There were also enlarged lymph nodes potentially signalling metastatic disease in the right neck levels 1B and 2A measuring up to 18 mm in diameter. In the context of this clinico-radiologic presentation, the lesion was suspicious for a sarcoma, a benign mesenchymal tumor or a lymphoma. The incisional biopsy displayed a low-grade myofibroblastic neoplasm with mild atypia, initially suspicious for a low grade sarcoma given the aggressive clinical features. Additional immunohistochemical stains were ordered; the lesion stained positive for SMA, desmin, caldesmon, and ß-catenin and negative for MUC-4, AE/AE3, myogenin, SOX-10, S100, and CD34. This pattern was most suggestive of DF, however complete extirpation would be required for a definitive diagnosis. A fluorodeoxyglucose (FDG)-positron emission tomography (PET) scan was ordered which demonstrated an intensely FDG avid (SUV 9.5) mass of the masticator space with no evidence of distant metastasis. (Fig. 2)
Fig. 1

Fig. 2A

2A, 2B. FDG-PET/CT scan demonstrating a FDG avid mass (SUV 9.5) of the masticator space 2C.
Fig. 2B

2A, 2B. FDG-PET/CT scan demonstrating a FDG avid mass (SUV 9.5) of the masticator space 2C.
Fig. 2C

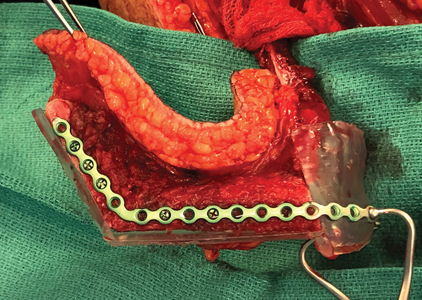
Following presentation at the Sarcoma Tumor Board, the patient underwent a right masticator space compartment resection including a composite mandibulectomy with condylar disarticulation, partial maxillectomy, and a superselective neck dissection of levels Ia, Ib, and IIa. (Fig. 3) Frozen sections were sent intraoperatively to evaluate the margins and assess the level I and II lymph nodes which were suspicious on pre-operative imaging. All margins and nodes were found to be negative for tumor intra-operatively. The patient was reconstructed with a right free vascularized fibula flap which was planned using in-house virtual surgical planning with printed mandibular and fibular cutting guides. (Fig. 4) The fibula was placed 3-4 mm superior to inferior border of the
mandible to aid in future dental implant rehabilitation.
Fig. 3A
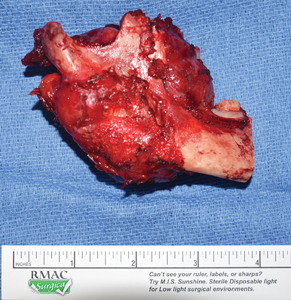
Fig. 3B

Fig. 4A

Fig. 4B
The excised specimen revealed a fairly well circumscribed firm fibrous tumor, which measured 6.5 x 6.1 x 3.4 cm and had a tan trabecular cut surface. The tumor infiltrated the surrounding skeletal muscle, adipose tissue and the mandibular ramus. Histologically it displayed long sweeping fascicles of bland spindle myofibroblasts in a collagenous stroma. Gaping thin-walled blood vessels were also present throughout the tumor. These findings confirmed the diagnosis of DF. Complete excision with negative margins was limited by the internal carotid artery posteriorly and the skull base superiorly, resulting in microscopic involvement of the posterior and superior margins. (Fig. 5) The tumor did not involve the inferior alveolar nerve and all lymph nodes were benign.
Fig. 5A

It infiltrates into the adipose tissue and skeletal muscle 5C. and the mandibular ramus 5D.
Fig. 5B
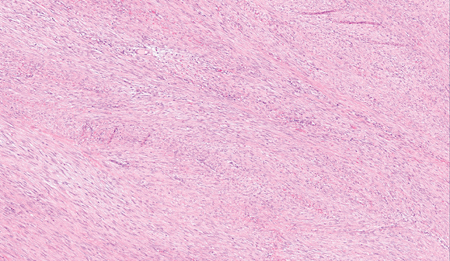
It infiltrates into the adipose tissue and skeletal muscle 5C. and the mandibular ramus 5D.
Fig. 5C

It infiltrates into the adipose tissue and skeletal muscle 5C. and the mandibular ramus 5D.
FIg. 5D
Her post-operative course was complicated by an upper gastrointestinal bleed, which was felt to be secondary to nasogastric tube insertion trauma or excessive non-steroidal anti-inflammatory (NSAID) use pre-operatively. She underwent vessel ligation, epinephrine injection, and cauterization by the gastroenterology team on post-operative day seven and was kept on intravenous pantoprazole for 72 hours prior to discharge. The remainder of her post-operative course was unremarkable. At one month post-operatively all of her wounds were healed and she had MIO of greater than 40 mm with maintenance of her pre-operative occlusion. (Fig. 6)
Fig. 6

She was presented to the Sarcoma Tumor Board and the decision was made to withhold adjuvant intensity modulated radiotherapy (IMRT) given her young age, and the large field required with anticipated significant morbidity and the lack of consensus in the literature on the significance of microscopically-involved margins – with several studies suggesting a minimally increased risk of recurrence.5,8 She is scheduled to be surveilled with MRIs every 3 months for the first year, with increasing intervals of follow-up thereafter. If recurrence is detected at the skull base, she is planned to receive small field targeted radiation using stereotactic body radiation therapy (SBRT). At four months post-operatively she will undergo soft tissue flap debulking, dental implant placement, and scar revision of her cervical and lower extremity scars.
Discussion:
Due to its rarity and aggressive clinical and radiologic presentations in this location, DTs can pose a diagnostic challenge as demonstrated in this case. According to Penel et al.6 one-third of DTs can be initially misdiagnosed. As such, they recommend for these cases getting a second opinion by a pathologist specializing in mesenchymal tumors. The case discussed in this article was initially thought to be a low-grade myofibroblastic sarcoma. The presence of spindle-shaped cells with collagen stroma adds to the complexity of the diagnosis, making it difficult to differentiate between sarcoma and DT. Immunohistochemistry stains can aid in the diagnosis, as overexpression of ß-catenin is usually present in 80% of DTs, although it is not specific and can be found in other soft tissue tumors.3,6
There are two main types of DT: sporadic and associated with familial adenomatous polyposis (FAP). The latter constitutes approximately 10% of DTs. In 90% of cases, the sporadic type is associated with somatic mutations of CTNNB1 gene, while the FAP type is caused by germline mutations of the APC gene.6,7
It has been reported that trauma can be a trigger to the development of DTs. In fact, 19-49% of DT cases developed after the site was subjected to trauma.8 In this case, extraction of tooth #48 preceded the event of trismus. However, the radiolucent lesion in the right ramus was evident prior to the extraction. The nuance of the faint lesion was only noticed after the patient had experienced symptoms. Other causes of DTs include genetics and hormones, as DTs have been reported in pregnant individuals and younger individuals during their puberty.3 It has been found that the etiology related to the increase in tumor size after biopsy is attributed to bleeding from the biopsy site rather than actual tumor growth.9
The newly published global consensus-based guidelines for the management of DTs regard “active surveillance” as the primary management strategy for asymptomatic DTs.9 The literature suggests that the event-free survival rate with surveillance and intervention appears to be similar; a subset of DTs in fact regress or remain stable over time. As such, for some patients, an initial period of active surveillance may spare them the morbidity of an intervention without compromising disease control.
In cases of disease progression, the presence of symptoms, or when the tumor is located in a site where further progression may lead to significant morbidity or mortality, intervention is required. Active management strategies include surgery, radiotherapy, medical management, which includes NSAIDS, anti-hormonal therapy, chemotherapy, or multimodality treatment.9 Unfortunately, only low-level evidence is available to guide clinicians. The choice of modality is dependent on the risk profile of the intervention, the tumor’s location and individual patient characteristics such as age or co-morbidities. For instance, in the case of abdominal wall DTs, which may often be removed with minimal morbidity, a surgical approach may be preferred, whereas in tumor locations where surgery may result in significant functional or cosmetic sequalae, medical therapy or radiotherapy may be preferred.9
In this present case, the patient was symptomatic with trismus, pain, and lingual nerve paresthesia. Moreover, the tumor was abutting the internal carotid posteriorly, the skull base superiorly, and causing some deviation of the airway. As such, it was felt that with any tumor progression there was the potential for life-threatening complications. Furthermore, the histopathological diagnosis was not certain based on the incisional biopsy. As such, an initial surgical approach was decided upon to both obtain a definitive diagnosis and for treatment. On final pathology, there were microscopically involved margins (R1 resection) at the skull base and along the carotid artery. Given the infiltrative nature of the tumor, the anatomical limitations of access to the masticator space, and the desire to minimize morbidity, a high rate of positive margins has been described in the literature (45-62%) for head and neck DFs.3 Interestingly, there is conflicting evidence, however, on the implications of leaving microscopic disease and the risk of local recurrence,3,5,9,10 with several studies indicating a minimal increased risk of recurrence. The benefit of adding adjuvant radiotherapy is also uncertain with studies reporting a reduction in the absolute risk of recurrence after surgery with the addition of radiotherapy (37% versus 25%) not reaching statistical significance (RR 0.69, 95% CI 0.41-1.17). In the present case, given the uncertain benefit and the morbidity of large field radiotherapy in a young patient, the decision of the multidisciplinary tumor board was to omit adjuvant radiotherapy and closely observe the patient. In the event of recurrences at the skull base, targeted small field radiation using SBRT is planned.
Conclusion:
DTs are rare and locally aggressive neoplasms that may occur in the maxillofacial region. As seen above, both the diagnosis and treatment are often a significant challenge. As such, DTs are best treated in high-volume centers with experience managing DTs and in conjunction with a multidisciplinary team.
Oral Health welcomes this original article.
References
- Wu C, Nik-Amini S, Nadesan P, Stanford WL, Alman BA. Aggressive fibromatosis (desmoid tumor) is derived from mesenchymal progenitor cells. Cancer Res. 2010;70(19):7690–8.
- Penel N, Coindre JM, Bonvalot S, Italiano A, Neuville A, Le Cesne A, et al. Management of desmoid tumours: A nationwide survey of labelled reference centre networks in France. Eur J Cancer. 2016;58:90–6.
- Eelco de Bree 1, Odysseas Zoras, Jennifer L Hunt, Robert P Takes, Carlos Suárez, William M Mendenhall, Michael L Hinni, Juan P Rodrigo, Ashok R Shaha, Alessandra Rinaldo, Alfio Ferlito R de B. Desmoid tumors of the head and neck: a therapeutic challenge. Head Neck. 2014;36(10):1517–26.
- Shields CJ, Winter DC, Kirwan WO, Redmond HP. Desmoid tumours. Eur J Surg Oncol [Internet]. 2001;27(8):701–6. Available from: https://pubmed.ncbi.nlm.nih.gov/11735163/
- Sharma A, Ngan BY, Sándor GKB, Campisi P, Forte V. Pediatric aggressive fibromatosis of the head and neck: a 20-year retrospective review. J Pediatr Surg. 2008;43(9):1596–604.
- Penel N, Chibon F, Salas S. Adult desmoid tumors: Biology, management and ongoing trials. Curr Opin Oncol. 2017;29(4):268–74.
- Baranov E, Hornick JL. Soft Tissue Special Issue: Fibroblastic and Myofibroblastic Neoplasms of the Head and Neck. Head Neck Pathol. 2020;(14):43–58.
- Goellner JR, Soule EH. Desmoid tumors. An ultrastructural study of eight cases. Hum Pathol. 1980;11(1):43–50.
- Alman B, Attia S, Baumgarten C, Benson C, Blay JY, Bonvalot S, et al. The management of desmoid tumours: A joint global consensus-based guideline approach for adult and paediatric patients. Eur J Cancer [Internet]. Elsevier Ltd; 2020;127:96–107. Available from: https://doi.org/10.1016/j.ejca.2019.11.013
- Mathijs P Hendriks, Chantal M L Driessen, Hanneke W M van Laarhoven, Geert O R J Janssens, Berit M Verbist, Winette T A van der Graaf, Piet J Slootweg, Matthias A W Merkx CML van H. Aggressive fibromatosis in the head and neck region: Benign tumor with often mutilating effects. Head Neck. 2013;35(8):246–50.
About the Author
 Dr. Baabdullah is a third year resident in oral and maxillofacial surgery at McGill University. She completed her dental degree at King AbdulAziz University in Saudi Arabia. She then completed a year of surgical internship at McGill University.
Dr. Baabdullah is a third year resident in oral and maxillofacial surgery at McGill University. She completed her dental degree at King AbdulAziz University in Saudi Arabia. She then completed a year of surgical internship at McGill University.
 Dr. Gigliotti is a specialist in oral and maxillofacial surgery. He is an Assistant Professor and an attending surgeon at the McGill University Health Centre in Montreal, Canada. He completed his dental degree at the University of Manitoba, and his medical degree and residency in oral and maxillofacial surgery at McGill University. He then completed a fellowship in head and neck oncology and microvascular reconstructive surgery at the University of Alabama at Birmingham/O’Neal Comprehensive Cancer Center.
Dr. Gigliotti is a specialist in oral and maxillofacial surgery. He is an Assistant Professor and an attending surgeon at the McGill University Health Centre in Montreal, Canada. He completed his dental degree at the University of Manitoba, and his medical degree and residency in oral and maxillofacial surgery at McGill University. He then completed a fellowship in head and neck oncology and microvascular reconstructive surgery at the University of Alabama at Birmingham/O’Neal Comprehensive Cancer Center.
 Dr. Makhoul is a specialist in oral and maxillofacial surgery with sub-specialization in Maxillofacial Oncology and Microvascular Reconstructive Surgery. He is an associate professor and an attending surgeon at the McGill University Health Centre. Dr. Makhoul is also an associate dean of postgraduate dental education and director of the department of dentistry.
Dr. Makhoul is a specialist in oral and maxillofacial surgery with sub-specialization in Maxillofacial Oncology and Microvascular Reconstructive Surgery. He is an associate professor and an attending surgeon at the McGill University Health Centre. Dr. Makhoul is also an associate dean of postgraduate dental education and director of the department of dentistry.
 Dr. Jung is an attending pathologist specialized in bone and soft tissue pathology and dermatopathology as well as assistant professor at McGill Medical School.
Dr. Jung is an attending pathologist specialized in bone and soft tissue pathology and dermatopathology as well as assistant professor at McGill Medical School.













