Oral leukoplakia is defined by the World Health Organization (WHO) Collaborating Centre in 2020 as “White plaques of questionable risk having excluded (other) known diseases that carry no increased risk for cancer”.1 It is the most common oral pre-cancerous lesion and, by definition, oral leukoplakia excludes white lesions such as frictional keratoses, namely benign alveolar ridge keratosis, morsicatio buccarum, and other specific diagnoses such as leukoedema, oral hairy leukoplakia and candidiasis to name a few.2
The most common sites for oral leukoplakia are the tongue (36–50%), buccal mucosa (18–26%), floor of the mouth (8–22%), gingiva (6–22%), and mucosa of hard palate (7%).3 Common sites for malignant transformation are the tongue, buccal mucosa, gingiva, and floor of the mouth, and 4–20% of gingival leukoplakias undergo malignant transformation.4 Although the gingiva is not the most common site for leukoplakia, it is a site with a high recurrence rate of 42–53%.5 Among oral leukoplakias, there is a distinct pathological entity first described by Hansen et al. in 1985: proliferative verrucous leukoplakia (PVL). Its malignant transformation rate is the highest of all potentially malignant lesions in the oral cavity.6
Most authors estimate a ratio of women to men of 4:1. Conversely, Palaia et al. found that women with PVL were 1.7 times more likely to experience malignant transformation and transformation to squamous cell carcinoma is estimated to be 3 times higher for women than for men.7 The literature reports that PVL lesions are most commonly located in the gums and alveolar mucosa, followed by the jugal mucosa, the tongue, the palatal mucosa, the floor of the mouth and the lips. There is a sub-type of PVL called gingival PVL, which occurs around the marginal gingiva of teeth and is particularly common in the anterior region. The literature indicates that these lesions are multifocal in almost all patients. Most authors mention smoking habits in their cohort but seem to exclude smoking as a risk factor for developing PVL. The few studies reporting alcohol consumption in their patients agree it should be excluded from the etiological factors for the occurrence of PVL.
The abnormality of this lesion is its macroscopic variability according to its clinical stage and location. The different developmental stages of this disease can be observed at the same time, in the same patient and at different sites. The lesions tend to go through 4 main clinical stages. In the initial stages, we can see an isolated, homogeneous leukoplakia that is predominantly white or inhomogeneous, possibly with erythematous halo-like margins, thin or thick, with a smooth surface, non-protuberant, soft on palpation or verrucous and often asymptomatic. It does not disappear after removal of apparent traumatic causes, is persistent and does not detach when scraped. Gradually, the lesions multiply and become thick, exophytic, papillomatous and/or verrucous and hard upon palpation.8,9
Major and minor criteria have been proposed in connection with differential diagnosis of PVL.
Major criteria:
- lesion with at least 2 different sites in the oral cavity,
- presence of verrucous area
- changes in lesion size during the development
- recurrence in previously treated area.
Minor criteria:
- all areas together occupying at least 3 cm2 ,
- female patient,
- non-smoking patient,
- disease manifestation longer than 5 years.
Oral verrucous hyperplasia and verrucous carcinoma can be one of the intermediate stages of PVL but can also occur in isolation.
Given the fact that the proliferative verrucous hyperplasia is connected with high rate of malignant transformation, repeated histological examination and frequent re-evaluation, removal of complete lesion are the best prevention of possible complications. Surgical access includes classic use of scalpel, electrosurgery, cryotherapy and laser surgery. Surgical removal with scalpel seeks to totally remove lesions, which is rarely possible given the multifocality of the lesions and the tissue loss that this procedure involves. This procedure may often require additional treatments, advanced surgery with tissue flaps or skin grafts that cannot guarantee eradication of the disease. Excision of gingiva, especially presenting as extensive lesion, is very suitable for laser use. Lasers show advantages over classic surgery due to better visualization mediated by good hemostasis of surgical site and eliminating the need for sutures. Furthermore, universal is decontamination of surgical field and substantial reduction of postoperative pain and oedema.
There is insufficient scientific evidence to conclude that any treatment strategy is capable of reducing PVL recurrence rate.
Case report
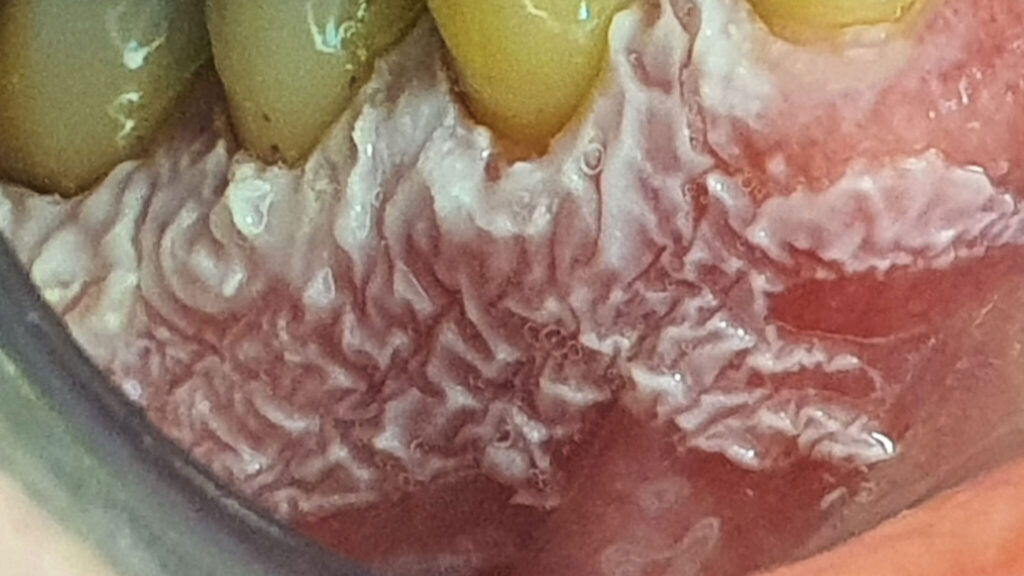
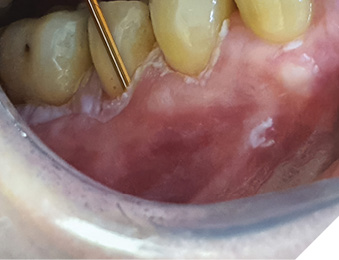
A 62 year-old-patient was referred for evaluation and treatment of white lesions affecting free and attached gingiva in all four quadrants. Generally healthy person and also history of smoking and alcohol drinking was negative. Periodontal examination revealed mild chronic gingivitis with localized manifestations of initial chronic periodontitis. Vestibular areas of premolar and molar regions were covered by white, mostly homogenous, verrucous plaques firmly connected with epithelial base. Detail of specific surface quality in lower right quadrant is visible in Figure 1.
Fig. 1
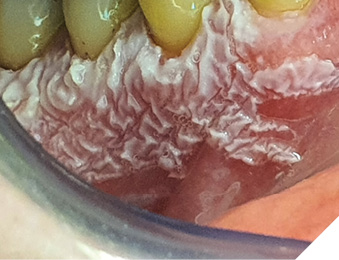
Under the diagnostic hypothesis of PVL, an incisional biopsy was taken in the anterior region of the lesion by conventional surgical technique under local infiltrative anesthesia with Ultracaine D-S forte ( Hoechst, Germany). Microscopic analysis showed typical corrugated hyperkeratosis with mild epithelial dysplasia. On the basis of the result of biological activity, the lesion was indicated for complete removal. For this purpose, 2780 nm Er,Cr:YSGG laser iPlus (Biolase, CA, USA) was used with 2,50 W power, 75 Hz frequency, H-mode (60 µs pulse duration), 20% air, 40% water and MC3 -9 mm tip. The procedure was performed without anesthesia (Fig. 2).
Fig. 2
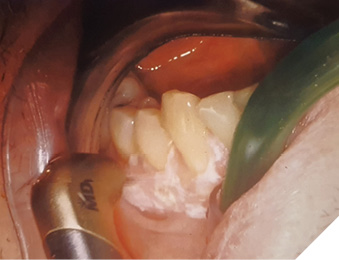
Final result of laser therapeutical ablation is visible in Figure 3.
Fig. 3

The course of healing was asymptomatic and comfortable. Next examination after 3 months revealed specific recurrency of hyperkeratosis affecting marginal part of previously treated gingiva (Fig. 4).
Fig. 4
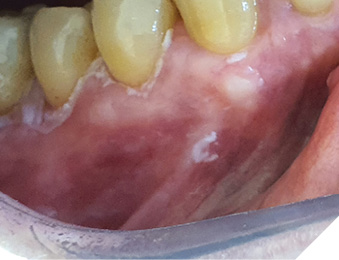
Arrangement of new hyperkeratotic lesion gave rise to presumption, that the source of recurrency could be invisible and untreated sulcular epithelium. One possible explanation for recurrence is that the complete removal of such gingival leukoplakias is almost impossible if the associated teeth are not removed, because of involvement of the sulcular epithelium so that any remnant dysplastic cells repopulate the oral surface of adjacent gingiva (Fig. 5).
Fig. 5
The subsequent step of the treatment was directed to detailed removal of persistent hyperkeratotic layers. Visible oral part of the lesion was removed using the same laser, according to the parameters already mentioned. Intrasulcular ablation of hyperkeratotic epithelium was mediated by the same laser device, but at different settings and using periodontal radial firing tip RFPT5-14 specifically designed for intrasulcular applications. Settings were adjusted to 1,50 W power, 30 Hz frequency, H-mode (60 µs pulse duration), 40% air and 50% water. The tip movement inside gingival sulcus was continual at velocity of 2 mm/s (Fig. 6).
Fig. 6
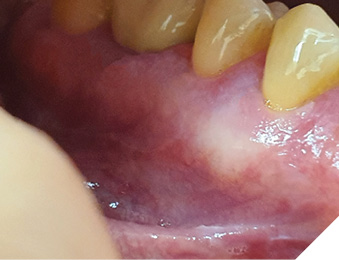
Healing process occured normally, the patient has been under follow-up, and no clinical signs of recurrence of the lesion were observed during subsequent 2 years (Fig. 7).
Fig. 7
Discussion
Different types of lasers have been described in connection with the treatment of oral hyperkeratotic lesions, such as CO2, Nd:YAG, Er:YAG or diode lasers, with very good results.10,11 But gingiva is specific oral region due to the very thin layer of soft tissue covering sensitive alveolar bone and especially marginal gingiva could be vulnerable in interaction with lasers showing deep penetration. Moreover, multiple layers of keratinized cells of leukoplakia are free of specific chromophores for diode lasers of NIR spectrum and hyperkeratotic tissue removal is not ablative but excisional which is connected with more significant loss of gingival tissue. This scenario could be unfavourable especially for thin gingival biotype. Er,Cr:YSGG laser is able to remove hyperkeratosis layer by layer without traumatic effect on underlying structures due to shallow penetration depth. This mechanism of laser ablation is also helpful for the visual control of tissue preparation. Complete removal of hyperkeratosis is essential step in succesful treatment and prevention of PVL recurrence.
It has been shown that the gingival connective tissue base will determine the characteristics of the overlying epithelium by carrying the genetic determinants for its specificity including keratinization.12 Since there is no apparent difference between the connective tissues underlying the sulcular and the outer gingival epithelium, it seems reasonable to assume that both epithelia, under the influence of the same connective tissue, may carry the same intrinsic potential for keratinization. The research findings indicate that the sulcular epithelium has potential for keratinization and its deepithelization is a delicate procedure incorporating the positive therapeutical effect on the one hand and possible destructive potential affecting the rest of marginal gingiva on the other hand. Bandara et al.13 described a case of PVL of the gingiva treated with CO2 laser ablation and the lesion recurred twice within a short time despite the surgical resection following the first recurrence. According to the presented pictures, clinical manifestation was nearly the same as in the case of our patient after first laser treatment, but the targeted removal of sulcular epithelium has not been mentioned.
Hypoxia-inducible factor-1α (HIF-1α), a glycoprotein with tyrosine kinase activity, plays a crucial role in progression of oral leukoplakias and is connected to the recurrence of PVL lesions. Consequently, HIF-1α levels are a reliable predictor of postoperative recurrence after ablative surgery. Previous studies have shown that erbium laser irradiation significantly downregulates the expression of HIF-1α and pro-inflammatory cytokines and promotes keratinization.14,15 By stimulating fibroblast proliferation, erbium laser irradiation enhances wound healing, ultimately reducing postoperative recurrence rates.16
Conclusion
In conclusion, Er,Cr:YSGG laser is efficient and safe device for ablative therapy of gingival PVL. Combined thermal and mechanical ablation mechanism constitutes rapid vaporization and micro-explosions due to pressure exceeding tissue structural integrity and achieving precise excision of the diseased tissue. The observed changes in the biochemical profile of treated tissue with subsequent inhibition of postoperative recurrence are promising, and require further investigation. 
Reprinted with permission from the Journal of Laser-Assisted Dentistry (JLAD).
References
- Warnakulasuriya S, Kujan O, Aguirre-Urizar JM, Bagan JV, González-Moles MÁ, Kerr AR, et al. Oral potentially malignant disorders: a consensus report from an international seminar on nomenclature and classification, convened by the WHO Collaborating Centre for Oral Cancer. Oral Dis. 2021;27(8):1862–80.
- Woo SB. Oral epithelial dysplasia and premalignancy. Head Neck Pathol. 2019;13(3):423-39.
- Dost F, Le Cao K, Ford PJ, Ades C, Farah CS. Malignant transformation of oral epithelial dysplasia: a real-world evaluation of histopathologic grading. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014;117(3):343–52.
- Goodson ML, Sloan P, Robinson CM, Cocks K, Thomson PJ. Oral precursor lesions and malignant transformation– who, where, what, and when? Br J Oral Maxillofac Surg. 2015;53(9):831–5.
- Kuribayashi Y, Tsushima F, Sato M, Morita K, Omura K. Recurrence patterns of oral leukoplakia after curative surgical resection: important factors that predict the risk of recurrence and malignancy. J Oral Pathol Med. 2012;41(9):682–8.
- Iocca O, Sollecito TP, Alawi F, Weinstein GS, Newman JG, De Virgilio A, et al. Potentially malignant disorders of the oral cavity and oral dysplasia: A systematic substantreview and meta-analysis of malignant transformation rate by subtype. Head Neck. 2020; 42: 539-55.
- Palaia G, Bellisario A, Pampena R, Pippi R, Romeo U. Oral proliferative verrucous leukoplakia: progression to malignancy and clinical implications. Systematic review and meta-analysis. Cancers. 2021; 13: 4085.
- Abadie WM, Partington EJ, Fowler CB, Schmalbach CE. Optimal management of proliferative verrucous leukoplakia: A systematic review of the literature. Otolaryngol Head Neck Surg. 2015; 153: 504-11.
- Staines K, Rogers H. Oral leukoplakia and proliferative verrucous leukoplakia: a review for dental practitioners. Br Dent J. 2017; 223: 655-61.
- Natekar M, Raghuveer HP, Rayapati DK, Shobha ES, Prashanth NT et al. A comparative evaluation: oral leukoplakia surgical management using diode laser, CO2 laser, and cryosurgery. J Clin Exp Dent. 2017; 9: 779-784.
- de Pauli Paglioni M, Migliorati CA, Faustino ISP, Mariz BALA, Roza ALOC, Vargas PA et al. Laser excision of oral leukoplakia: does it affect recurrence and malignant transformation? A systematic review and meta-analysis. Oral Oncol. 2020; 109:104850.
- Caffesse RG, Karring T, Nasjledti CE. Keratinizing potential of sulcular epithelium. J Periodontol. 1977;48(3):140-146.
- Bandara DL, Jayasooriya PR, Jaysinghe RD. Proliferative verrucous leukoplakia of the gingiva: An early lesion refractory to surgical excision. Case Rep Dent. 2019;2019:5785060.
- Rashid M, Zadeh LR, Baradaran B, et al. Up-down regulation of HIF-1α in cancer progression. Gene. 2021;798(9):145796.
- Aoki A, Mizutani K, Schwarz F, et al. Periodontal and peri-implant wound healing following laser therapy. Periodontol. 2015;68(1):217–69.
- Feist IS, De Micheli G, Carneiro SR, et al. Adhesion and growth of cultured human gingival fibroblasts on periodontally involved root surfaces treated by Er:YAG laser. J Periodontol. 2003;74(9):1368–75.
About the author

Dr. Pavel Polenik graduated from the Charles University Prague, Czech Republic and started his professional life as Assistant Professor at Dept. of Periodontology of Charles University , Medical Faculty Pilsen. After research fellowship at Aarhus University (Danmark) founded laboratory of Oral Biology with significant impact on contemporary research in the field of laser therapeutical methods. 23 years user of laser technologies of many wavelengths. Member of European Federation of Periodontology, member of WFLD, member of former ESOLA, graduate of ESOLA Laser Academy (Vienna, 2007), president of CZESOLA, mentor of oral laser application for oral laser users in Czech Republic.