Abstract
While several in vitro studies have focused on the antibacterial activity of root canal disinfection protocols and sealing ability of root filling materials, the exact clinical relevance remains unknown. In this pilot study, we aimed at identifying the possible causes of treatment failure in teeth that had been endodontically treated and diagnosed as treatment failures due to persistent pain. Teeth (n=12) diagnosed as failed root canal treatment were extracted when patients were unwilling for revision endodontics and analyzed histologically after staining using Taylor modified Brown and Brenn staining. Nine specimens demonstrated intraradicular bacteria and bacteria within the dentinal tubules while the voids between the root filling material and root canal wall (which was present in all specimens), as well as voids within the root filling material showed minimal or no bacteria. The results of the study also indicated the need for identifying extraradicular biofilms as potential causes of root canal treatment failure. Remnant bacteria within the root canal system appears to be the primary cause for treatment failure and methods should be developed to completely disrupt biofilms within the root canal system.
Introduction
The objective of root canal treatment is prevention or treatment of apical periodontitis.It has been reported that properly done root canal treatment results in healing of periradicular lesions by means of hard tissue regeneration.1,2 While the exact microbiology of primary and secondary infections remain unclear, it is evident that pulp and periradicular infections are biofilm mediated.
Few papers have addressed the possible causes of treatment failure using a histological approach.3,4 Nair, in his landmark review, concluded that the causes of root canal treatment failure could be categorized into microbial and non-microbial causes, with residual microbes within the root canal system being the major cause.5 Several studies have evaluated the antimicrobial activity of root canal disinfecting agents and methods. A recent systematic review demonstrated a clear lack of clinical trials on this topic and concluded that with the available evidence, there was no significant difference between sodium hypochlorite (NaOCl) and chlorhexidine in bringing about bacterial reduction or endotoxins.6 This is in contrast to several in vitro studies that show the superiority of NaOCl in bringing about biofilm disruption and reduction of bacterial counts.7-9
Leakage via the root canal system has been a topic of controversy for several years because of the lack of conclusive evidence that laboratory leakage can negatively impact clinical outcomes. Several studies have addressed leakage of root filling materials and it appears that all root fillings leak. The assumption that coronal and apical leakage can result in treatment failure is based on the premise that this can result in ingress of bacteria into the root canal space or can serve as a source of nutrition to the bacteria that were incompletely removed after root canal preparation.10,11 Whether the bacteria that are assumed to be entombed within the root filling material can repopulate on exposure to nutrition also appears to be unknown. While it is known that reduction of bacteria below a specific threshold results in healing12, such a threshold remains unidentified.
In this paper, we qualitatively determine the possible causes of failure of root canal treatment using histobacteriological methods.
Materials and Methods
This pilot study included patients who had persistent pain following root canal treatment. The study design was approved by the Institutional Review Board and Ethics Committee of the Saveetha University. The inclusion criteria were that: patients should be in good health, root canal treatment should have been performed less than five years prior, teeth should be periodontally sound. All patients were offered three treatment choices: non surgical root canal retreatment, surgical endodontics or extraction. Patients opting for extraction were enrolled in this study and 12 teeth were obtained. These teeth were extracted either because of the patient’s unwillingness to undergo retreatment or non-restorability. Informed consent was obtained from the patients. The sample size included 4 mandibular first molars, 2 mandibular second molars, 3 maxillary first molars, 3 mandibular first premolars.
Tissue Processing
The teeth were immersed in 10% neutral-buffered formalin for at least 48 hours immedately after removal. Specimens were demineralized in a aqueous mixture of 22.5% (vol/vol) formic acid and 10% (wt/vol) sodium citrate for three to four weeks, with the endpoint being determined radiographically. All specimens were washed in running tap water for 24-48 hours, dehydrated in ascending grades of ethanol, cleared in xylene, infiltrated, and embedded in paraffin according to standard procedures. Using a microtome, sections with a thickness of 4-5 microns were obtained parallel to the long axis of the root canal. Only the apical segment was evaluated in this study. Random sections from each specimen were stained with Taylor modified Brown and Brenn technique for staining bacteria.13 The slides were analyzed for the following features: presence of bacteria within the dentinal tubules, presence of spaces between the root canal filling and root canal wall, presence of remnant pulp tissue in the root canal space, presence of bacteria in the spaces between the root canal filling and root canal wall, presence of voids within the root canal filling and microbial presence within these voids, debris and microbial presence in the apical foraminal region and lateral canals.
Results
Representative histological images and their interpretations have been shown in Figures 1-6. The presence or absence of the specific findings listed in the Materials and methods has been tabulated (Table 1). Specimen 1 (Fig. 1) showed numerous bacterials masses within the dentinal tubules. These were mostly gram-negative bacteria (A). Spaces were present between the root filling and root dentin (B) and these spaces showed dense bacterial biomasses, which were mostly gram positive (C). There were several voids within the root filling material, but no microbes were found here (D). There were also areas of possible pulp tissue remnants. Three of the 12 specimens showed numerous gram-negative bacterial masses within the dentinal tubules, possibly extending to the external root surface. These bacteria were noticed as dense clumps in the areas close to the root canal wall. Light microscopy could not be used to differentiate between cocci and bacilli.
Table 1

Figures 1-3

Representative histological images of teeth evaluated in this study demonstrating the possible causes of failure of treatment. The description can be found in the results section.
Specimen 2 (Fig. 2) demonstrated very minimal bacteria within the dentinal tubules (A) with spaces between the root filling and dentin (B) as well as voids within the root filling (C) showing no bacterial masses. This specimen also showed possible pulp tissue remnants (D). Specimen 3 (Fig. 3) showed minimally infected dentinal tubules (A) with spaces between the root filling and dentin (B) as well as the voids within the root filling demonstrating gram positive bacteria (B).
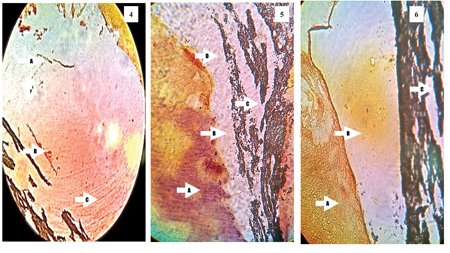
Figures 4-6
Figures 7-9
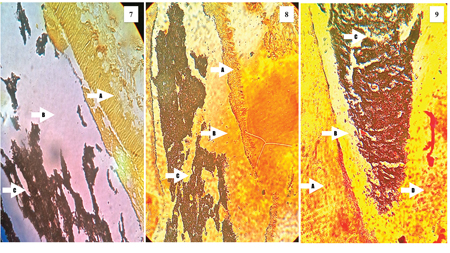
Representative histological images of teeth evaluated in this study demonstrating the possible causes of failure of treatment. The description can be found in the results section.
Specimen 4 (Fig. 4) showed numerous gram positive (A) and gram negative bacteria (B) within the dentinal tubules, with gram negative bacteria extending along the entire length of the dentinal tubules length that was assessed (C). Specimen 5 (Fig. 5) demonstrated numerous gram negative bacteria within the dentinal tubules (A), spaces between (B) and within the root filling (C) showing no microbial masses with possible pulp tissue remnants (D). Specimen 6 (Fig. 6) showed relatively clean dentinal tubules with minimal or no bacterial masses (A). The spaces between the root filling and dentin (B) as well as voids within the root filling (C) has no microbial masses. The findings in specimen 7 (Fig. 7) were similar to specimen 6.
Specimen 8 (Fig. 8) showed numerous bacteria limited to the part of the dentinal tubules close to the root canal space (A), while the spaces between the root filling and dentin (B) as well as voids within the root filling material (C) showed no microbial masses. Specimen 9 (Fig. 9) was that of a C-shaped canal system and demonstrated gram negative bacteria extending along the entire length of the specimen examined (A) with spaces between the root filling and dentin (B) and within the root filling (C) showed no microbes. Pulp tissue remnants were also found in this specimen (D). Specimen 10 (Fig. 10) shows the isthmus area in the mesial root of a maxillary molar which was filled with pulp tissue (A) and partially filled with the root filling material (B). The isthmus region showed gram-negative bacterial masses (C) while the dentinal tubules showed gram-positive bacteria (D).
Figures 10-12
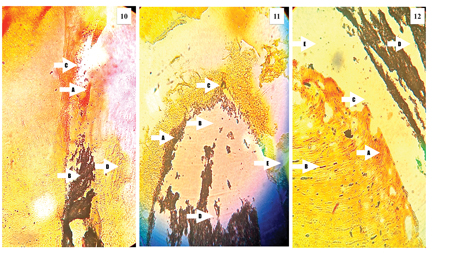
Representative histological images of teeth evaluated in this study demonstrating the possible causes of failure of treatment. The description can be found in the results section.
Specimen 11 (Fig. 11) showed numerous gram positive bacterial masses (A) in the spaces between the root filling and dentin, as well as the spaces between the root filling and apical foraminal region (B). The apical foraminal region was blocked with debris containing gram positive and gram-negative bacteria (C). The voids within the root filling had no bacteria (D). The lateral canals were filled with debris but contained no bacteria (E). Specimen 12 (Fig. 12) showed numerous gram negative bacterial biofilms in the root canal wall closer to the root canal space (A), with gram positive bacteria being present within the dentinal tubules (B), extending along the length of the specimen examined. The voids between the root filling material and dentin (B) as well as the voids within the root filling (C) showed no microbial masses. The ramifications in the apical third (D) showed no microbes.
Discussion
The present pilot study was performed to qualitatively characterise the conditions that possibly resulted in failure of root canal treatment. While the exact mechanisms for successful outcomes have not been clearly elucidated, cleaning and shaping of the root canal system with an adequate sealing appear to be factors that favor successful outcomes of treatment.14 Whether each of these parameters can be singled out to have significant impact on outcomes is unknown and this study aimed to find an answer to this question.
All the specimens examined in this study showed voids between the root filling material and root dentin. It is not known if this was because of an improper obturation technique or dissolution of sealer. While several studies have been published on the leakage of root filling materials using different models, its clinical validity has been questioned.15 In general, this leakage could be corono-apical or apico-coronal. The term coronal leakage refers to any entry of microbiota into a root filled tooth so as to set a host response in the periradicular tissues. In contrast, apical leakage implies the entry of microbes or microbial products into the apical third of the root canal system from the periradicular region, serving as a new source of microbes or as a source of nutrition to the microbes that remain within the root canal system.16
The results of the present study showed that only two of the 12 specimens examined demonstrated bacteria in the space between the root filling and dentin i.e., 83.4% of the samples showed the no bacteria in these spaces, which are potential leakage pathways. This makes one question the clinical relevance of “sealing ability” of root canal filling materials and techniques. A very important finding in this study was the presence of bacteria within the dentinal tubules. Nine of the 12 specimens showed bacteria within the dentinal tubules. Two of these specimens also had spaces between the root filling and dentin filled with bacteria. However, it is difficult to address if the bacteria found in these two specimens were present after root canal treatment or they entered via the leakage route. In all the specimens bacteria could be seen along the entire length of the areas analyzed.
Biofilm bacteria demonstrate upto 1000 fold resistance to antimicrobial agents, compared to planktonic suspensions.17 For a root canal irrigant to be clinical effective, it is necessary that it has the ability to disrupt microbial biofilms in addition to demonstrating antibacterial activity.18 Dentinal tubule disinfection and antibiofilm activity of irrigation regimes and techniques within the dentinal tubules has been studied extensively.19-21 These studies show that syringe irrigation of irrigants is the least effective in bringing about intratubular disinfection while activation methods such as passive ultrasonic irrigation, sonic activation, laser activation and light activated disinfection offer superior results albeit variability in different studies. The irrigating solution also appears to play an important role. In vitro evidence consistently shows that sodium hypochlorite (NaOCl) in varying concentrations shows superior antibacterial and antibiofilm activity compared to saline and antiseptics such as chlorhexidine. Furthermore, NaOCl is the only irrigant capable of dissolving vital and necrotic pulp tissue.7-9,22 However, methodological issues could have a bearing on the clinical applicability of these results: (i) Most in vitro studies evaluate the efficacy of irrigating solutions and techniques on artificially created biofilms of Enterococcus fecalis and (ii) most studies only evaluate the antibacterial activity without attributing the relevance to biofilms.
With regards to the first methodological issue, Enterococcus fecalis, a gram positive cocci has been reported to be the most commonly isolated bacteria in failed root canal treatment cases.23,24 This species has been reported to be able to survive extremely harsh environments and hence, withstand intracanal treatment procedures.25,26 However, the present study showed both gram positive and gram-negative bacteria within the root canal space as well as the dentinal tubules. This is in accordance with recent molecular microbiological methods that have elucidated that the microbial biofilm within the root canal system is complex and may contain more than one species, the characteristics of which are very different from single species biofilms used in in vitro models.17,27 Primary root infected specimens have been shown the presence of at least seven phyla (Firmicutes, Bacteroidetes, Actinobacteria, Proteobacteria, Fusobacteria, Synergistetes and Spirochaetes).28 This emphasises the fact that antibiofilm studies should use multispecies biofilms to make the results clinically relevant.
One of the specimens in this study showed that the apical foramen was blocked with debris, and also contained several microbial masses. The concept of patency filing so as to convert the close-ended root canal system to an open ended system, and thereby allow better exchange of irrigants at the apical third has found a controversial place in endodontics.29 Machado et al. reported that patency filing may not always be necessary based on analysis of the risks and benefits. But the authors also stated that there was no clinical evidence to correlate apical patency to outcome of treatment.30 It is possible that this specimen failed because of blockage of the apical foraminal region with debris and lack of irrigant exchange at this region resulting in persistent infection.
One interesting finding in this study was that four specimens demonstrated very minimal or no bacteria within the root canal system as well as within the dentinal tubules. This calls into question, the possibility of considering extra radicular infection as a possible cause for root canal treatment failure. Studies in the past have evaluated the reasons for root canal treatment failure.31-33 These studies did not report intra-radicular infection as a major cause of root canal treatment failure. Nair et al. contradicted these reports and stated that this could be because of methodological errors.34 The findings of Nair et al. cannot be ignored. However, the possibility of extra radicular infection in four specimens that showed failure of treatment in this study cannot be over ruled. Periapical actinomyces could be the potential cause of treatment failure in such cases because Actinomyces has been shown to establish extraradicularly and penetrate the root canal space even in teeth with good orthograde treatment.35,36 This needs further analysis.
In conclusion, this pilot study showed that intraradicular and intratubular bacteria are the primary cause for root canal treatment failure although the possibility of extra radicular infection cannot be ruled out. Clinical procedures must focus on the delivery methods and activation of root canal irrigants to efficiently disrupt biofilms within the root canal system to improve clinical outcomes. OH
Disclosure: The authors declare no conflict of interest.
Oral Health welcomes this original article.
References
- Strindberg LZ. The dependence of the results of pulp therapy on certain factors. An analytic study based on radiographic and clinical follow-up examinations. Acta Odontologica Scandinavica 1956;14: 1–175.
- Sundqvist G, Figdor D, Persson S, Sjogren U. Microbiologic analysis of teeth with failed endodontic treatment and the outcome of conservative re-treatment. Oral Surgery, Oral Medicine and Oral Pathology 1998;85: 86–93.
- Ricucci D, Siqueira JF Jr, Bate AL, Pitt Ford TR .Histologic investigation of root canal-treated teeth with apical periodontitis: a retrospective study from twenty-four patients. Journal of Endodontics 2009;35:493-502.
- Ricucci D, Siqueira JF Jr. Biofilms and apical periodontitis: study of prevalence and association with clinical and histopathologic findings. Journal of Endodontics 2010; 36:1277-1288.
- Nair PN. On the causes of persistent apical periodontitis: a review. International Endodontic Journal 2006; 39:249-281.
- Gonçalves LS, Rodrigues RC, Andrade Junior CV, Soares RG, Vettore MV. The effect of sodium hypochlorite and chlorhexidine as irrigant solutions for root canal disinfection: A systematic
review of clinical trials. Journal of Endodontics 2016;42:527-532. - Ordinola-Zapata R, Bramante CM, Cavenago B, Graeff MS,
Gomes de Moraes I, Marciano M, Duarte MA. Antimicrobial effect of endodontic solutions used as final irrigants on a dentine biofilm model. International Endodontic Journal 2012;45:162-168. - Wang Z, Shen Y, Haapasalo M. Effectiveness of endodontic
disinfecting solutions against young and old Enterococcus faecalis biofilms in dentin canals. Journal of Endodontics 2012; 38:1376-9. - Zehnder M. Root canal irrigants. Journal of Endodontics 2006; 32:389-98.
- Tabassum S, Khan FR. Failure of endodontic treatment: the usual suspects. European Journal of Dentistry 2016; 10:144-7.
- Siqueira JF Jr, Rôças IN, Ricucci D, Hülsmann M. Causes and management of post-treatment apical periodontitis. British Dental Journal 2014;216:305-12.
- Siqueira JF Jr, Rôças IN. Microbiology and treatment of acute apical abscesses. Clinical Microbiology Reviews 2013; 26:255-73.
- Siqueira JF Jr, Rôças IN. Clinical implications and microbiology of bacterial persistence after treatment procedures. Journal of Endodontics 2008;34:1291-1301.
- Siqueira JF. Aetiology of root canal treatment failure: Why well-treated teeth can fail. International Endodontic Journal 2001; 34:1–10.
- Editorial Board of the Journal of Endodontics (2007) Wanted: a base of evidence. Journal of Endodontics 33, 1401–2.
- Verissimo DM, do Vale MS. Methodologies for assessment of apical and coronal leakage of endodontic filling materials: a critical review. Journal of Oral Science 2006;48:93-98.
- Svensäter G, Bergenholtz G. Biofilms in endodontic infections.Endod Topics 2004;9:27—36.
- Ordinola-Zapata R, Bramante CM, Aprecio RM, Handysides R,
Jaramillo DE. Biofilm removal by 6% sodium hypochlorite activated by different irrigation techniques. International Endodontic Journal 2014;47:659-666. - Cachovan G, Schiffner U, Altenhof S, Guentsch A, Pfister W, Eick S. Comparative antibacterial efficacies of hydrodynamic and ultrasonic irrigation systems in vitro. Journal of Endodontics 2013; 39: 1171-1175.
- Halford A, Ohl CD, Azarpazhooh A, Basrani B, Friedman S, Kishen A. Synergistic effect of microbubble emulsion and sonic or ultrasonic agitation on endodontic biofilm in vitro. Journal of Endodontics 2012;38:1530-4.
- Neelakantan P, Cheng CQ, Ravichandran V, Mao T, Sriraman P, Sridharan S, Subbarao C, Sharma S, Kishen A. Photoactivation of curcumin and sodium hypochlorite to enhance antibiofilm efficacy in root canal. dentin. Photodiagnosis and Photodynamic Therapy 2015;12:108-114
- Haapasalo M, Shen Y, Qian W, Gao Y. Irrigation in endodontics. Dental Clinics of North America 2010; 54: 291-312.
- Pinheiro ET, Gomes BPFA, Ferraz CCR, Sousa ELR, Teixeira FB, Souza Filho FJ. Microorganisms from canals of root-filled teeth with periapical lesions. International Endodontic Journal 2003;36:1–11.
- Fouad AF, Zerella J, Barry J, Spangberg LS. Molecular detection of Enterococcus species in root canals of therapy-resistant endodontic infections. Oral Surgery Oral Medicine Oral Pathology Oral Radiology Endodontology 2005;99:112– 8.
- Evans M, Davies JK, Sundqvist G, Figdor D. Mechanisms involved in the resistance of Enterococcus faecalis to calcium hydroxide. International Endodontic Journal 2002;35:221– 8.
- Love RM. Enterococcus faecalis: a mechanism for its role in endodontic failure. International Endodontic Journal 2001;34:399 – 405.
- Chavez de Paz LE. Development of a multispecies biofilm community by four root canal bacteria. Journal of Endodontics 2012; 38:318-323.
- Svensäter G, Bergenholtz G. Biofilms in endodontic infections. Endodontic Topics 2004;9:27—36.
- Ribeiro AC, Matarazzo F, Faveri M, Zezell DM, Mayer MPA. Exploring bacterial diversity of endodontic microbiota by cloning and sequencing 16s rRNA. Journal of Endodontics 2011; 37:922-926.
- Souza RA. The importance of apical patency and cleaning of the apical foramen on root canal preparation. Brazilian Dental Journal 2006;17:6-9.
- Machado R, Ferrari CH, Back E, Comparin D, Tomazinho LF,
Vansan LP. The impact of apical patency in the success of endodontic treatment of necrotic teeth with apical periodontitis: a brief review. Iranian Endodontic Journal 2016; 11: 63-66. - Seltzer S, Bender IB, Smith J, Freedman I, Nazimov H. Endodontic failures – an analysis based on clinical, Roentgenographic, and histologic findings. Parts I & II. Oral Surgery Oral Medicine Oral Pathology 1967;23:500-530.
- Andreasen JO, Rud J. A histobacteriologic study of dental and periapical structures after endodontic surgery. International Journal of Oral Surgery 1972;1:272-281.
- Block RM, Bushell A, Rodrigues H, Langeland K. A histopathologic, histobacteriologic, and radiographic study of periapical endodontic surgical specimens. Oral Surgery Oral Medicine Oral Pathology 1976;42:656-678.
- Nair PNR, Sjögren U, Kahnberg KE, Krey G, Sundqvist G. Intraradicular bacteria and fungi in root-filled, asymptomatic human teeth with therapy-resistant periapical lesions: a long- term light and electron microscopic follow-up study. Journal of Endodontics 1990;16:580-588.
- Sundqvist G, Reuterving CO. Isolation of Actinomyces israelii from periapical lesion. Journal of Endodontics 1980;6:602-606.
- Nair PNR, Schroeder HE. Periapical actinomycosis. Journal of Endodontics 1984;10:567-570.
 Gayathri Mohan is an undergraduate student at Saveetha Dental College and Hospitals, Saveetha University, Chennai, India. This work was carried out in Saveetha Dental College and Hospitals, Chennai.
Gayathri Mohan is an undergraduate student at Saveetha Dental College and Hospitals, Saveetha University, Chennai, India. This work was carried out in Saveetha Dental College and Hospitals, Chennai.
 Nithya Jagannathan is a former Assistant Professor of Oral and Maxillofacial Pathology, Saveetha Dental College and Hospitals, India.She is now a Research Associate at the Faculty of Dentistry, The University of Hong Kong, Hong Kong SAR.
Nithya Jagannathan is a former Assistant Professor of Oral and Maxillofacial Pathology, Saveetha Dental College and Hospitals, India.She is now a Research Associate at the Faculty of Dentistry, The University of Hong Kong, Hong Kong SAR.
 Dr. Prasanna Neelakantan is a former Assistant Professor of Endodontics, Saveetha Dental College and Hospitals, Chennai. Currently he holds the position of Clinical Assistant Professor of Endodontology at the Faculty of Dentistry, The University of Hong Kong.
Dr. Prasanna Neelakantan is a former Assistant Professor of Endodontics, Saveetha Dental College and Hospitals, Chennai. Currently he holds the position of Clinical Assistant Professor of Endodontology at the Faculty of Dentistry, The University of Hong Kong.
RELATED ARTICLE: Root Canal Treatment of Maxillary Premolars With Three Roots: A Case Series












