
Periodontal disease (gingivitis and periodontitis) is an oral inflammatory condition that is an all too common oral problem.1 Gingivitis often presents as bleeding, swollen gums, and pain, and if left untreated progresses to periodontitis, which involves the loss of periodontal attachment and supporting bone. The imbalance between oral bacteria and the innate immune cells, particularly oral neutrophils, holds significant importance in the progression of the disease.2 Once triggered by an accumulation of pathogenic bacteria, the oral neutrophils migrate into the gingival crevice to eradicate these bacteria, preventing their penetration into deeper tissues. If the situation is unresolved, chronic oral inflammation generated by oral neutrophils ultimately results in tissue destruction, the hallmark of periodontal diseases.3
Given the high prevalence of periodontal disease, the significant impact it has on society and individuals, and the availability of effective prevention and treatment, why is there such a gap between the prevalence of the disease and the level of treatment? Missed and delayed diagnosis and inadequate treatment lead to more severe forms of periodontal diseases along with tooth loss. Even among patients of record who diligently maintain regular dental visits, barriers to early diagnosis and treatment of periodontal disease persist. The relationship between periodontitis and systemic health has become an increasingly important area of study in recent years, shedding light on the potential far-reaching impacts of this oral condition on overall well-being. Our recent research has hinted at a potential link between periodontal disease and the metabolic control of diabetes in diabetic patients.4 Furthermore, several studies have corroborated an association between periodontal disease and oral squamous cell carcinoma, suggesting that the unique immunological environment found within periodontal diseases may play a role in facilitating tumor progression.5 In addition to these findings, investigations have shown that periodontal diseases can lead a systemic hyperinflammatory state.6 This suggests that periodontal tissue inflammation may exert systemic effects, potentially influencing interactions with other inflammatory conditions.
A striking parallel can be drawn between the prevention of periodontal disease and the prevention of stroke, prior to the introduction of a straightforward method for measuring blood pressure. Both conditions share commonalities in terms of their gradual development, which makes establishing precise early diagnoses time-consuming and monitoring treatment efficacy challenging, as well as inflicting substantial societal burdens with irreversible consequences. In the medical field, the innovation of the modern sphygmomanometer (blood pressure cuff) played a pivotal role in identifying and managing hypertension, thus helping many individuals avert strokes. Prior to the year 1900, physicians faced difficulties in identifying individuals with elevated blood pressure,7 analogous to the challenges dental professionals currently encounter when assessing oral inflammation associated with periodontal disease.
Within the realm of dentistry, conventional techniques for detecting oral inflammation rely heavily on invasive and subjective clinical assessments. These methods include visually inspecting gingival tissues to evaluate the presence or absence of gingival inflammation8 (assessing color and tissue swelling) and conducting preliminary assessments of oral hygiene (evaluating plaque and calculus levels). Subsequent steps involve measuring probing depths. However, recording comprehensive periodontal charts for periodontally healthy patients during each visit proves excessively time-intensive and labor-demanding, potentially deterring patients from seeking dental care. Additionally, challenges in reproducing precise measurement placements, forces, and angles, along with the limited sensitivity of radiographs, underscore the need for more objective, minimally invasive, and less technique-dependent screening approaches for oral inflammation.9–11 Dentists and hygienists require more sophisticated tools to assess and monitor oral inflammation precisely. These advanced tools would enable timely intervention at the earliest signs of disease, facilitate ongoing evaluation of treatment efficacy, and provide patients with tangible evidence of their oral inflammation levels, thereby promoting acceptance and adherence to their treatment regimen.
A promising method for evaluating oral inflammation involves measuring oral neutrophils, which can indicate the presence of inflammation in the mouth.12 This line of research has yielded interesting findings that could reshape how we assess oral health. By using a quick and simple 30-second oral rinse, researchers have observed changes in oral neutrophil counts that align with the degree of inflammation AND correlate with the severity of periodontal diseases, as determined by commonly accepted clinical standards. For example, oral neutrophil counts have been found to correlate with the degree of bleeding on probing and probing depth13 the levels of oral neutrophils. In fact, another intriguing discovery comes from investigating how periodontal treatment impacts oral neutrophil counts. Individuals who respond positively to periodontal treatments demonstrated significant reductions in oral neutrophil counts.12 This not only underscores the potential effectiveness of periodontal treatment but also suggests that oral neutrophil counts could be a valuable biomarker for tracking improvements in oral health. The cumulative evidence from these studies indicates that utilizing simple oral rinses to measure oral neutrophil counts could potentially provide a reliable method for screening for periodontal inflammatory diseases. As ongoing research explores this area, it is increasingly compelling to establish how oral neutrophil measurements might become a standard part of evaluating oral health.
While the prospect of employing oral neutrophils as biomarkers for various health conditions holds considerable promise, the traditional methods to measure oral neutrophils have always required specialized labs, expensive equipment and trained personnel. This has made it less suitable for widespread use in dental clinics. To address these limitations, an innovative solution to simplify the process of oral neutrophil-based diagnostics and make it more accessible and useful in various healthcare settings was required.
In response to the need for a simple, rapid, precise, and engaging tool, PerioMonitorTM was developed. PerioMonitorTM is a point-of-care (POC) device designed to meet the need for a rapid, cost-efficient, and user-friendly test for the detection of oral inflammation levels in clinical settings. The test utilizes a simple, colorimetric approach based on the enzymatic activity of oral neutrophils that are secreted during the inflammatory response.
This simple rapid colorimetric test is administered by having the patient rinse their mouth with 10 mL of a solution for 30 seconds and then expectorate the oral rinse into a clean test container. At the chair, the dental professional then dips the test strip into the oral rinse sample for 1 second and waits for 1 minute while the color changes from clear to varying shades of purple. The resulting color is compared to a chart to interpret the results. This color chart is carefully calibrated to correlate with established concentrations of oral neutrophils and levels of oral inflammation. (Fig. 1)
Fig. 1

Fig. 2
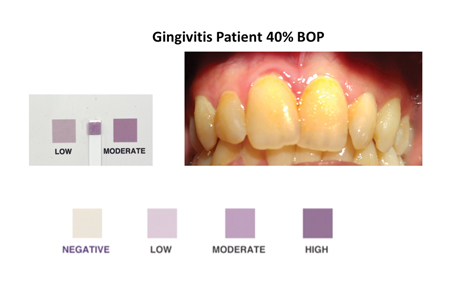
Fig. 3
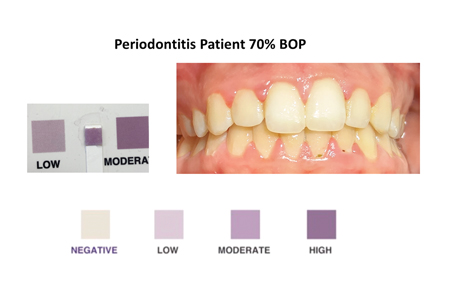
What sets this test apart is its exceptional accessibility and simplicity, which require minimal operator involvement. This streamlined procedure eliminates the need for complex laboratory setups and specialized personnel, making it highly adaptable for integration into a diverse range of clinical settings. The results can then be shown to the patient to increase understanding and participation in their diagnosis and monitoring of treatment success. This unique feature of engaging patients directly in their periodontal disease diagnosis and management aligns with the trend towards patient involvement in healthcare decisions.
PerioMonitorTM holds substantial potential to reshape the landscape of clinical diagnostics. In a society where the swift and accurate identification of dental disease is expected, this test presents an interesting avenue of exploration. Its ability to rapidly detect oral inflammation at the chair with dental patients, or at the bed among institutionalized elderly patients, through a non-invasive procedure administered by non-specialized staff, could have significant implications for healthcare practices. Recent research highlights the applicability of such an approach in diverse clinical contexts, especially in patient groups that are traditionally challenging to work with. For example, it can be used with uncooperative adults with special needs14 and pregnant females15 who need frequent monitoring, reinforcing the test’s potential. Taken together, these findings suggest PerioMonitorTM’s potential to reshape the screening and diagnosis of periodontal diseases, particularly in cases where time is of the essence, patient compliance is challenging, or frequent monitoring is needed.
Beyond its established applications in dentistry, the innovative oral rinse test, developed for the quantification of oral neutrophils, offers intriguing prospects for expansion into wider healthcare settings. This test’s unique ability to objectively and rapidly assess oral inflammation has recently been leveraged to establish a direct link between gingival health and cardiovascular function16. Notably, its capacity to predict vascular dysfunction suggests its potential to identify individuals at risk, including those who are young and seemingly healthy. The test’s association with endothelial dysfunction, an early marker for cardiovascular disease, hints at the possibility of a non-invasive approach to screening for cardiovascular health. By introducing this pioneering methodology, new opportunities arise for interdisciplinary collaborations and further research aimed at verifying the test’s efficacy in detecting systemic health risks beyond the confines of dentistry.
In conclusion, just as the simple Blood Pressure Cuff has become indispensable for physicians managing hypertension, PerioMonitorTM may prove to be an equally indispensable tool for dental professionals in their efforts to diagnose periodontal diseases, monitor treatment effectiveness, track disease progression, and deliver proactive patient care to everyone. The device will enhance clinical decision-making, optimize treatment outcomes, and contribute to the overall well-being of patients. PerioMonitorTM is currently [under review] by the FDA and Health Canada as a chair-side device for use in dental clinics by either dentists, hygienists, or dental assistants (www.periomonitor.com). As we embrace this innovative approach, the path forward lies in streamlining its integration through collaboration among dental practitioners, researchers, and technological experts. By uniting innovation and accessibility, periodontal disease diagnosis and healthcare can be revolutionized.
Oral Health welcomes this original article.
References
- Kinane DF, Stathopoulou PG, Papapanou PN. Periodontal diseases. Nature Reviews Disease Primers. 2017;3(1):17038. doi:10.1038/nrdp.2017.38
- Scott DA, Krauss J. Neutrophils in periodontal inflammation. Front Oral Biol. 2012;15:56-83. doi:10.1159/000329672
- Aboodi GM, Goldberg MB, Glogauer M. Refractory Periodontitis Population Characterized by a Hyperactive Oral Neutrophil Phenotype. Journal of Periodontology. 2011;82(5):726-733. doi:10.1902/jop.2010.100508
- Lakschevitz F, Aboodi G, Tenenbaum H, Glogauer M. Diabetes and Periodontal Diseases: Interplay and Links. Current Diabetes Reviews. 2011;7(6):433-439. doi:10.2174/157339911797579205
- Elebyary O, Barbour A, Fine N, Tenenbaum HC, Glogauer M. The Crossroads of Periodontitis and Oral Squamous Cell Carcinoma: Immune Implications and Tumor Promoting Capacities. Frontiers in Oral Health. 2021;1. https://www.frontiersin.org/articles/10.3389/froh.2020.584705
- Fine N, Chadwick JW, Sun C, et al. Periodontal Inflammation Primes the Systemic Innate Immune Response. J Dent Res. 2021;100(3):318-325. doi:10.1177/0022034520963710
- Kotchen TA. Historical trends and milestones in hypertension research: a model of the process of translational research. Hypertension. 2011;58(4):522-538. doi:10.1161/HYPERTENSIONAHA.111.177766
- Meitner SW, Zander HA, Iker HP, Polson AM. Identification of inflamed gingival surfaces. Journal of Clinical Periodontology. 1979;6(2):93-97. doi:10.1111/j.1600-051X.1979.tb02187.x
- Canakci V, Canakci CF. Pain levels in patients during periodontal probing and mechanical non-surgical therapy. Clin Oral Investig. 2007;11(4):377-383. doi:10.1007/s00784-007-0126-z
- Karayiannis A, Lang NP, Joss A, Nyman S. Bleeding on probing as it relates to probing pressure and gingival health in patients with a reduced but healthy periodontium. A clinical study. J Clin Periodontol. 1992;19(7):471-475. doi:10.1111/j.1600-051x.1992.tb01159.x
- Lang NP, Nyman S, Senn C, Joss A. Bleeding on probing as it relates to probing pressure and gingival health. Journal of Clinical Periodontology. 1991;18(4):257-261. doi:10.1111/j.1600-051X.1991.tb00424.x
- Bender JS, Thang H, Glogauer M. Novel rinse assay for the quantification of oral neutrophils and the monitoring of chronic periodontal disease. Journal of Periodontal Research. 2006;41(3):214-220. doi:10.1111/j.1600-0765.2005.00861.x
- Landzberg M, Doering H, Aboodi GM, Tenenbaum HC, Glogauer M. Quantifying oral inflammatory load: oral neutrophil counts in periodontal health and disease. Journal of Periodontal Research. 2015;50(3):330-336. doi:10.1111/jre.12211
- Moosani A, Sigal MJ, Glogauer M, Lawrence HP, Goldberg M, Tenenbaum HC. Evaluation of periodontal disease and oral inflammatory load in adults with special needs using oral neutrophil quantification. Special Care in Dentistry. 2014;34(6):303-312. doi:10.1111/scd.12077
- Huda S, Doering H, Tenenbaum H, Whittle W, Sigal M, Glogauer M. Oral Neutrophil Levels: A Screening Test for Oral Inflammatory Load in Pregnancy in a Medical Setting. Journal of periodontology. 2014;86:1-17. doi:10.1902/jop.2014.140116
- Hong KY, Ghafari A, Mei Y, et al. Oral inflammatory load predicts vascular function in a young adult population: a pilot study. Frontiers in Oral Health. 2023;4. https://www.frontiersin.org/articles/10.3389/froh.2023.1233881
About the Authors

Dr. Omnia Elebyary is a third-year PhD student at the Faculty of Dentistry, University of Toronto. Her research focuses on discovering oral biomarkers for systemicdiseases, emphasizing oral health’s role in holistic healthcare.

Dr. Michael Glogauer is an internationally recognized clinician-scientist and leader in the fields of neutrophil biology, innate immunity, oral microbiome, and periodontology. He is a Fellow of the Canadian Academy of Health Sciences. Dr. Glogauer is also a Professor at the Faculty of Dentistry, University of Toronto (since 2002), Head of Dentistry for University Health Network and Chief of Dental Oncology at Princess Margaret Cancer Centre.









