
BACKGROUND
- Medication prescribing is usually based on clinical trials and what has typically worked best for each healthcare provider (HCP) in the past.
- One-size-fits-all approaches are based on averages and do not account for individual responses due to genetic variations.1
- Therefore, drugs and doses that work well for one patient can often be ineffective, cause unexpected side effects, or even be unsafe for another.
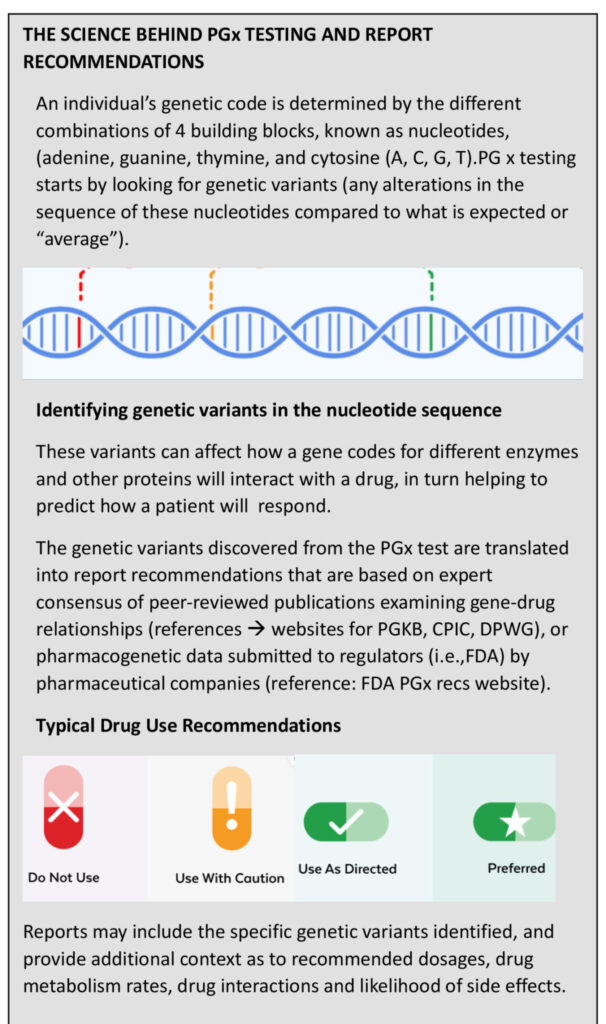
Pharmacogenetic Testing for Better Treatment Outcomes
Pharmacogenetics (PGx) is the combination of pharmacology (the study of drugs) and genetics (the study of individual, inherited traits). Pharmacogenetic testing identifies which gene variants an individual has inherited, and what that means in terms of which drugs and doses are likely to work best for them.
(PGx) testing reduces the trial and error of prescribing for dentistry by revealing which medications used in dentistry for pain management, anesthetics, inflammation, and sedation are likely to respond well for a patient, based on the specific genetic variants he or she carries. Using PGx test results to guide treatment can help dentists provide personalized care for their patients by reducing adverse effects and increasing treatment effectiveness.2
PGx Testing Procedure
The typical steps are as follows:
- The patient orders a PGx test kit (typically online) or purchases one from a HCP that has partnered with a service provider
- Upon receiving the test kit, the patient most commonly provides a cheek swap sample to gather the DNA required for analysis.
- The swab is sealed and sent to the testing lab.
- The lab’s runs the DNA sample through specialized equipment.
- The data results are then translated into a Results and Recommendations report.
- The patient receives the report (in a printable format and/or online) and can consent to share the results with their HCP’s.
What to look for when recommending a PGx Test Provider
- Testing provided by experts in the Pharmacogenetics field and that follow existing guidelines. Find out how results are translated into prescribing recommendations.
- Results delivered quickly to ensure that results are available to the dentist to refer to prior to patient’s scheduled surgery.
- Affordability, to increase patient accessibility. It should be noted however that PGx testing is covered by many health insurance plans which reduces cost objections.
- Simple and easy to interpret reports that can be easily shared between the patient and dentist. This enables quick action on the results without necessitating the additional intervention of the PGx company to first interpret and translate them.
- Reports that include direct links to all data sources, which provides transparent and easy access to recommendations, evidence, and additional contextual information.
- Timely Access to Support for answers to questions beyond the scope of the healthcare provider (dentist).
How to introduce PGx to your Patients
The preferred time to introduce PGx testing to patients is during the treatment plan consultation appointment so that results are available for reference prior to the next appointment for surgery. It also provides the opportunity to easily bundle (or include) the cost of testing in the treatment plan and make it more palatable for the patient. Many patient’s healthcare insurance plans fully or partially cover the cost of PGx testing.3
At patient checkout, staff can explain the simple PGx testing procedure (that typically takes less than a minute to follow) and reinforce the benefits. If staff also collect test samples from patients before they leave the office, it provides a much faster test report turnaround time since the onus is no longer on patients to remember to take the test and then send their samples to the lab.
Benefits to Patients
- Medications are prescribed based on the unique genetic profile of patients and therefore they experience better treatment outcomes.
- Reduces the risk of major adverse reactions by 30%.1
- Receive a comprehensive medication
- response profile that they can share with their chosen healthcare practitioners.
- Take charge of the management of their own personal healthcare from increased openness and adherence to new medications.
- Reduce time off work, disability claims and overall healthcare costs.4,5,6
Benefits to the Practice
- Builds practice loyalty by empowering patients with new knowledge about their healthcare. PGx testing reports on many other drugs beyond the scope of dentistry.
- Differentiates your dental practice by building and offering expertise/capabilities in a rapidly emerging market (personalized medicine prescribing).
- Creates and enhances collaboration/interprofessional relationships with other healthcare providers/prescribers (physicians and pharmacists).
- Generates new revenue streams from referral incentives offered by PGx testing service providers.
Published Evidence
The dental profession is known for insisting on the application of evidence-based science when evaluating the validity of improved treatment protocols and dental technology claims. Pharmacogenetic testing as it applies to dentistry should be no exception. Key evidence is summarized below.
Opioids: As per late 2022 review, individuals with specific genetic variants resulting in poor activity of the CYP2D6 hepatic enzyme could not adequately convert codeine and tramadol to their active metabolites, resulting in poor pain control. Conversely, those with specific genetic variants resulting in increased activity of the CYP2D6 hepatic enzyme over produced active metabolites, resulting in increased frequency of adverse events, including respiratory depression.7
Additionally, a landmark pharmacogenetic study (including nearly 7000 participants across several European countries) published in early 2023 found that employing pharmacogenetic testing to guide treatment resulted in 30% reduced risk of adverse effects attributable to genetic variants. Results were pooled across a number of medications in various therapeutic contexts, though codeine was well represented (tramadol and oxycodone to a lesser extent).1
NSAIDs: Evidence exists to support pharmacogenetic influence on pharmacokinetic (i.e., “what the body does to the drug”) parameters of NSAIDs (e.g., ibuprofen), including metabolism and clearance. Though additional studies need to be done to fully establish the impact of safety and efficacy in patients with genetic variants, much of the data that exists is actionable, and can primarily help guide dosing to reduce unnecessary overexposure (thereby minimizing risk of adverse effects).8,9
Anesthetics: Identifying patients with specific genetic variants that increase the risk of malignant hyperthermia secondary to the use of volatile, general anesthetics (including desflurane, isoflurane, sevoflurane) is supported by actionable insight from publications.10 In addition, drug manufacturer supplied data for local anesthetics (including mepivacaine, bupivacaine, lidocaine) carrying a risk of methemoglobinemia due to G6PD deficiency also informs pharmacogenetic testing.11
Diazepam: Drug manufacturer supplied data for the sedative diazepam highlights that some individuals with genetic variants may be susceptible to increased plasma concentrations of the medication,11 which could impact patient response.
Proton Pump Inhibitors: Pharmacogenetic testing can help identify patients that may require higher doses of proton pump inhibitors (e.g., pantoprazole, lansoprazole) secondary to oral sequelae due to unmanaged gastroesophageal reflux disease (e.g., dental erosion, gingivitis).12,13
Future Perspectives
Many drugs used for non-dental related indications have relevance to dental professionals. A growing list of these drugs have strong pharmacogenetic data, but the ramifications in oral health have yet to be explicitly determined or inferred by expert consortia. Given the rapid pace of discovery in pharmacogenetics, however, it wouldn’t be surprising if future insights build on what is already known and provide tangible recommendations that may be useful in various dental contexts. For example, we may see data that helps explain anticholinergic adverse effects such as xerostomia, or gingival hyperplasia secondary to antiseizure medication use. Additionally, pharmacogenetic insights about a patient’s bleeding risk based on their pre-existing use of various agents could also develop (e.g., anti-thrombotics, serotonergic antidepressants, anti-inflammatories).
Real life Dental Practice Experience offering PGx Testing
Dr. Robert Cappell, dentist and co-founder of Dentistry In Motion Toronto, is an early adopter of Inagene’s test to guide treatment in his dental practice. Says Dr. Cappell, “Dental procedures necessitate the use of anesthetics, anti-inflammatory, pain drugs and possibly oral sedatives. Suboptimal pain management can impair healing and delay recovery, and cause issues with eating, talking, and sleeping.” And it’s more than just the drugs administered at the dentist’s office. “Eighty percent of those visiting the dentist take one or more drugs, and a third take more than five. If drugs being taken for underlying medical conditions are not working well, it can impact oral health and can ultimately impair dentists’ abilities to deliver optimal care. Having medication that is personalized for patients will be of benefit to both of us.”
Dr. Natalie Wong, prosthodontist and Founder and Director of the Toronto Implant Institute Inc., pairs restorative procedures and surgery with personalized prescribing; medication management guided by PGx testing. “By combining our treatment plans with pharmacogenetic testing, our aim is to make what was previously unpredictable, predictable for patients” says Wong. “With a quick cheek swab test standard with many of our procedures, it allows us to tailor use medications during and after surgery to the patient’s unique genetics, to minimize pain and promote faster healing.”
Conclusion and Final Thoughts
It should be emphasized that PGx is simply a treatment decision aid tool and is not intended to replace professional judgement.5 That said, PGx Testing brings innovation to dental care that involves less pain during and after procedures, fewer safety risks and complications.
Beyond the scope of dentistry, PGx benefits society overall with the ability to lower group benefits costs, (particularly with respect to mental health and pain), by preventing drug wastage and reducing disability claims.6 The benefits of PGx testing also last a lifetime since one’s DNA profile never changes. As more research and clinical trials are conducted, the repository of PGX drug response recommendations will likely increase and continue to improve personalized prescribing efficacy.)
Oral Health welcomes this original article.
References
- Swen JJ, van der Wouden CH, Manson LE, et al. A 12-gene pharmacogenetic panel to prevent adverse drug reactions: an open-label, multicentre, controlled, cluster-randomised crossover implementation study. Lancet. 2023;401(10374):347-356. doi:10.1016/S0140-6736(22)01841-4.
- Wiley F. The Benefits of Pharmacogenetic Testing. Managed Healthcare Executive, January, 2020 Vol. 3, Issue 1.
- Kylie M. Long-Term Disability and Pharmacogenomic Testing, Resolute Legal Blog,Dec. 13, 2022.
- Koufaki MI, Fragoulakis V, Díaz-Villamarín X, et al. Economic evaluation of pharmacogenomic-guided antiplatelet treatment in Spanish patients suffering from acute coronary syndrome participating in the U-PGx PREPARE study. Hum Genomics. 2023;17(1):51. Published 2023 Jun 7. doi:10.1186/s40246-023-00495-3Y.
- https://medlineplus.gov/lab-tests/pharmacogenetic-tests/
- Winner J, Allen JD, Altar CA, Spahic-Mihajlovic A. Psychiatric pharmacogenomics predicts health resource utilization of outpatients with anxiety and depression. Translational Psychiatry. 2013;3:e242.
- Nahid NA, Johnson JA. CYP2D6 pharmacogenetics and phenoconversion in personalized medicine. Expert Opin Drug Metab Toxicol. 2022;18(11):769-785. doi:10.1080/17425255.2022.2160317.
- Zobdeh F, Eremenko II, Akan MA, et al. Pharmacogenetics and Pain Treatment with a Focus on Non- Steroidal Anti-Inflammatory Drugs (NSAIDs) and Antidepressants: A Systematic Review. Pharmaceutics. 2022;14(6):1190. Published 2022 Jun 1. doi:10.3390/pharmaceutics14061190.
- Theken KN, Lee CR, Gong L, et al. Clinical Pharmacogenetics Implementation Consortium Guideline (CPIC) for CYP2C9 and Nonsteroidal Anti-Inflammatory Drugs. Clin Pharmacol Ther. 2020;108(2):191-200. doi:10.1002/cpt.1830.
- Gonsalves SG, Dirksen RT, Sangkuhl K, et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for the Use of Potent Volatile Anesthetic Agents and Succinylcholine in the Context of RYR1 or CACNA1S Genotypes. [Supplement to: Clin Pharmacol Ther. 2019;105(6):1338-1344. doi:10.1002/cpt.1319].
- U.S. Food and Drug Administration. Table of Pharmacogenomic Biomarkers in Drug Labeling. https://www.fda.gov/drugs/science-and-research-drugs/table-pharmacogenomic-biomarkers-drug-labeling. Accessed [Date Accessed, e.g., August 22, 2023].
- Eken E, Estores DS, Cicali EJ, Wiisanen KK, Johnson JA. A Pharmacogenetics-Based Approach to Managing Gastroesophageal Reflux Disease: Current Perspectives and Future Steps. Pharmgenomics Pers Med. 2023;16:645-664. Published 2023 Jun 23. doi:10.2147/PGPM.S371994.
- Mahajan R, Kulkarni R, Stoopler ET. Gastroesophageal reflux disease and oral health: A narrative review. Spec Care Dentist. 2022;42(6):555-564. doi:10.1111/scd.12726.
About the Author

Dr. Natalie Wong graduated from the University of Toronto with her Doctor of Dental Surgery in 1996 and received her Certificate in Prosthodontics from the University of Michigan, Ann Arbor in 2007. She is Founder and Director of the Toronto Implant Institute Inc. and lectures nationally and internationally on implant dentistry while maintaining a private implant surgical and prosthodontic practice in Toronto.

Ashwin Juneja, MBA, PharmD, previously, a Pharmacy Manager dedicated to medication management, Ashwin now acts as Clinical Affairs Lead for Inagene Diagnostics Inc. Ashwin holds a Bachelor’s Degree in Pharmacy from University of Toronto, an Executive MBA from Queen’s University and a Doctorate in Pharmacy from U. of Toronto.

David Rajczak, founder of DJR Consulting, is a healthcare practice management and technology focused expert with over two decades of experience working with industry leaders such as ABELDent, Henry Schein and Inagene. He holds a Bachelor of Commerce degree and an MBA from McMaster University in Hamilton, Ontario. He can be contacted at drajczak@inagene.com or 905-577-5252.












