If you are reading this article, you were likely born in a country and era where medical testing is a routine part of healthcare. From birth, data collection begins with tracking weight and height, followed by simple blood tests to screen for potential concerns. As we progress through life, additional tests are conducted based on signs and symptoms, reflecting a reactive approach essential for treating existing conditions. On the other hand, some tests are used proactively to intercept diseases before they fully develop, allowing for early intervention and ultimately preventing disease.
Partnering with a clinical laboratory in the medical field is the result of a long and deliberate process. It began with the development of the technology, followed by its integration into educational institutions, and eventually, the adoption of testing as a standard of care. This evolution has spanned decades, if not a century. While some tests have been seamlessly integrated, others are gradually gaining acceptance in emerging areas like functional medicine and dentistry.
The dental clinician’s familiarity with saliva has made the collection of salivary specimens more straightforward. However, much like in the medical field, challenges remain. The adoption of this practice as a standard of care will take time. The good news is that as mounting evidence links oral health to overall health, partnering with a clinical laboratory will propel dentistry toward proactive, evidence-based care rather than reactive treatment. This collaboration will also foster more effective communication with other healthcare professionals.
The drive to deepen our understanding has been the catalyst for the evolution of modern periodontology. Universities and leading researchers began with culture testing, and through their efforts and advancements in molecular analysis, dental salivary diagnostics have become commercially viable. This progress not only transformed dentistry but also provided medical professionals with insights into oral health conditions. While it’s remarkable how far we’ve come, this is just a glimpse of where we are headed.
As more oral health care providers incorporate salivary testing into their protocols, a common question arises: “How will testing change my therapy?” This question can be interpreted in two ways.
First, “change my therapy” could mean, “What tools or periodontal adjuncts should I use?” Testing undoubtedly influences the choice of tools and treatments. This reactive approach provides an evidence-based foundation for treatment planning. For example, if a profile reveals bacteria with virulence factors and tissue invasiveness, and you have been practicing a ‘one-size-fits-all’ approach where every patient receives the same treatment, testing allows you to create a personalized plan targeting specific bacteria.
The second interpretation of “change my therapy” might be, “Why should I test when I already know periodontal disease is bacterial?” While it is true that bacteria are often a factor in periodontal disease, this assumption is not always accurate or the primary cause. Testing confirms the cause—are bacteria levels elevated? If so, which is often the case, testing validates the bacterial cause and guides targeted treatment. However, if bacteria levels are minimal, the treatment approach shifts to consider other factors like genetics, systemic conditions, lifestyle choices, and more. Without testing, treatment relies heavily on guesswork and assumptions, hoping the patient responds well.
A third interpretation involves a proactive approach. By testing before clinical signs and symptoms of periodontal disease appear, testing serves as a risk assessment tool. If elevated bacteria levels are detected, proactive intervention may prevent the development of periodontal disease.
Given the high prevalence of periodontal disease, most patients presented with testing are already in advanced stages. The ultimate goal of testing should be to intercept the disease process early by using testing as a screening tool. However, for this article, a classic example is used to demonstrate how testing impacted periodontal care, including implant placement, along with the benefits to the patient’s medical conditions. This case, navigated by Dr. Ashley Spooner, follows the patient from 2015 to today.
Periodontal case study
This classic periodontal case study begins in 2015. A traditional approach of scaling and root planing (SRP), irrigation and laser decontamination was performed, and the patient entered periodontal maintenance. With the sporadic commitment to home care, there were times of significant inflammation with minimal plaque and calculus, resulting in further tooth loss.
During a period of relapse (recurrence of periodontal disease), a course of periodontal therapy was performed starting with the collection of an oral rinse sample for bacterial testing called OralDNA® labs (USA) MyPerioPath® (Fig. 1).
Fig. 1
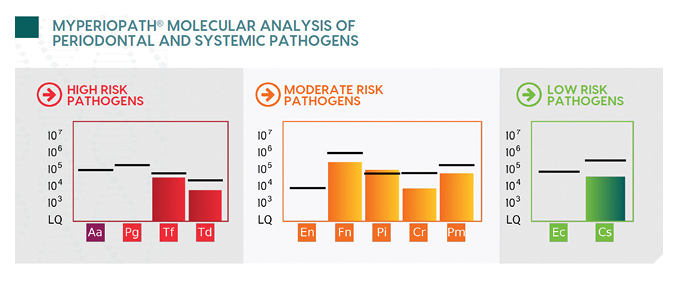
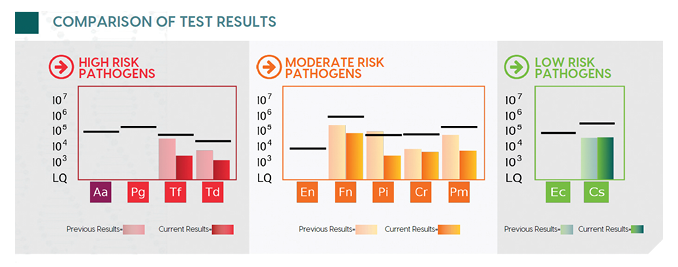
Sample collection involved a 30 second swish and gargle with sterile saline, followed by the addition of a preservative to the collected sample.1 The real-time quantitative polymerase chain reaction detected 7 of the 11 known periodontal pathogens, with their quantities indicated by the bar graph. Although the majority of the bacteria were below the reference line (benchmark for level of bacteria typically seen in patients with chronic periodontitis), the patient’s clinical signs of disease still indicated the patient needed therapy. Lower levels of bacteria lead to the potential for other complicating factors such as genetics, lifestyle choices, and/or systemic health. The presence of resistant/virulent/tissue invasive bacteria Tf, Td, Pi and Pm2 supported the use of SRP, irrigation, and laser decontamination, in addition to a course of systemic antibiotics. Metronidazole was the antibiotic chosen based on the bacterial profile.3,4 This is an example of how salivary testing can impact and change the decided course of therapy. Instead of a routine choice of antibiotic, the report provided an evidence-based, personalized recommendation. The patient was allowed to heal, then a post-therapy MyPerioPath test, or MyPerioProgress®, was performed. Pre-test results are shown in the faded color bar graphs and the current levels are shown in the solid color bar graphs, with a reduction of 18% (Fig. 2). The absence of clinical signs along with the reduction of the bacterial load resulted in remission for this patient. All results were shared with the primary care physician, providing a common language and solidifying the oral health care practitioner as key to this patient’s health.
Fig. 2
In 2022, the patient’s chief concern was securing an implant to improve chewing function. However, the patient exhibited visible symptoms, including jaundice, swollen purple feet, and a rapid weight loss of 60 pounds over just 3-4 months. Upon further investigation, the clinician discovered the patient was also suffering from persistent diarrhea, severe fatigue, and the inability to work. This led to an urgent referral for medical and sleep apnea evaluation before the procedure.
Sharing of the test results provided ease of communication with the medical team. It was no longer a letter stating clinical signs, but an objective lab report. The local medical professionals facilitated prompt care, revealing that the patient’s blood sugars were over 800 – dangerously high. This led to the initial diabetes mellitus diagnosis along with localized edema, disorders of veins, acute systolic (congestive) heart failure, splenomegaly, other primary thrombocytopenia, anemia, abnormality of albumin, ulcerative colitis, diverticulitis, other specified abnormal immunological findings in serum, and cirrhosis of the liver. The physicians and patient were grateful for the attention to overall health. The interprofessional relationships established from this case have led to a deeper understanding of the oral systemic link by all involved.
The multidisciplinary patient care direction was to prioritize the medical ailments while sustaining regular dental visits. The health conditions were improving until the feeling-better patient decided to discontinue the prescribed Metformin in January 2023. After this, the patient experienced accelerating signs of inflammation. This was confirmed by an abnormal activated MMP8 (aMMP8) score of 78 ng/mL, obtained by collecting a saline water specimen and analyzed in the Dentognostics (Germany) ORALyzer EXPERT® chairside. aMMP8 testing quantifies any tissue destruction occurring in the mouth.5,6 The patient was instructed to work on home care, adding localized probiotics and PerioScience® (USA) AO gel. In addition, the urgency of an appointment with their physician for better management of blood sugars with medication was emphasized.
At the next 3-month re-care appointment, the decreased aMMP8 score of <20 mg/mL was a significant achievement. However, the even greater improvement was the reduction in A1C from 10.9 to 8, blood glucose below 200, CRP from 34.6 to 5.6, thrombocytopenia stability, healthy weight maintenance, management of cirrhosis, and normal bowels. Finally with the patient’s commitment, the time was right for implant placement.
In this case, the long-term evaluation and follow-up of dental patients through consistent periodontal charting, radiographs, intraoral photos, and comprehensive medical reviews at each visit, are crucial for improving overall health outcomes. The attention of the dental team, with the necessary referrals, led to better health for this patient. The collaborative approach, including continued care, objective saliva testing, and adjunctive therapies such as irrigation, bacterial decontamination, systemic antibiotics, localized probiotics, and AO gel, has significantly improved the patient’s oral and overall health. Sharing salivary testing results with the primary care physician was an integral component in this care model. This combined with patient compliance, resulted in positive outcomes. Although the patient continues to face challenges with diet and blood sugar management, his conditions have improved, and the dental team emphasizes the critical link between oral and overall health. This case highlights the importance of comprehensive medical history reviews, detailed oral examinations, and salivary testing in enhancing patient care. It also underscores the need for effective communication and collaboration between dental and medical teams to improve health outcomes in the community.
This is a prime example of how salivary diagnostics are transforming patient care in the oral health space. These services are not only advancing medical applications but also fostering greater synergy between the medical and dental professions.
In conclusion, as oral health care is increasingly recognized as a vital component of overall well-being, the oral health care professional is incorporating salivary diagnostics to know more and to provide a common language to communicate this oral systemic connection to their medical colleagues. This progression calls for a proactive approach in elevating the standard of oral care, where regular testing becomes a cornerstone of practice. By adopting this approach, oral health care providers not only contribute to the early detection and prevention of systemic diseases but also foster a more collaborative relationship with other healthcare professionals. Including a well-crafted cover letter that addresses the oral-systemic connection, along with pertinent lab results, in your communications with other healthcare professionals, establishes a shared language and understanding across medical disciplines.
Ultimately, this method ensures a more comprehensive approach to patient care, bridging the gap between dentistry and general medicine. By embracing this model, practitioners are not only enhancing the quality of care but are also upholding the true essence of dental medicine—caring for the whole patient, not just their teeth. 
Oral Health welcomes this original article.
References
- K. Boutaga, P. Savelkoul, E. Winkel, A.J. van Winkelhoff. Comparison of Subgingival Bacterial Sampling With Oral Lavage for Detection and Quantification of Periodontal Pathogens by Real-Time Polymerase Chain Reaction. Journal of Periodontology 78 (2007): 79-86.
- Periodontology 2000Volume 5, Issue 1 p. 78-111Microbial etiological agents of destructive periodontal diseases Anne D. Haffajee, Sigmund S. Socransky First published: June 1994 https://doi.org/10.1111/j.1600-0757.1994.tb00020.x https://onlinelibrary.wiley.com/doi/10.1111/j.1600-0757.1994.tb00020.x
- Systemic Antibiotics in Periodontics; Journal of Periodontology 2004; 75: 1553-1565
- Systemic antibiotics in the treatment of periodontal disease; Periodontology 2000; 28: (1), 106-176
- Sorsa, Timo et al. “Active MMP-8 (aMMP-8) as a Grading and Staging Biomarker in the Periodontitis Classification.” Diagnostics 10 (2020): 61.
- Räisänen, Ismo et al. “Active Matrix Metalloproteinase-8 Point-of-Care (PoC)/Chairside Mouthrinse Test vs. Bleeding on Probing in Diagnosing Subclinical Periodontitis in Adolescents.” Diagnostics 9 (2019): 34.
About the Authors

Dr. Ashley Spooner graduated from University of Colorado School of Dental Medicine and practices in Highlands Ranch, CO. She is a Diplomate of the American Board of Dental Sleep Medicine and founder of Dynamic Dental Sleep where she teaches sleep medicine. As an Epic Physician Builder, she is helping to optimize interprofessional collaborative workflows using Electronic Health Records.

Diane Larson holds an associate degree in Dental Hygiene from Northcentral Technical College and a bachelor’s degree in Dental Hygiene from Minnesota State University Mankato. Passionate about the oral-systemic connection, she joined OralDNA in 2009, driven by her interest in salivary testing. She has been involved with professional organizations like AAOSH and ADHA/MnDHA.