Introduction
Neutrophils (Polymorphonuclear neutrophils or PMNs) are a highly abundant white blood cell type, that are the first cells recruited to acute sites of infection. Through their essential role in immune surveillance and maintenance of organismal homeostasis, PMNs innately recognize tissue damage and infection, quickly home to relevant sites throughout the body and destroy invading pathogens. Although they have a short life span in the circulation, tissue neutrophils make up for this shortfall through their sheer abundance and their enormous tissue destructive capacity. Excessive neutrophil responses are frequently associated with tissue damage,1 which contributes to clinical manifestations in a wide array of chronic inflammatory diseases including periodontal disease.2,3 In such cases, where the initiation and recruitment of neutrophils, and therefore the magnitude of the neutrophil response, is not commensurate with the nature of the threat, neutrophils are a potential therapeutic target and also a potential diagnostic or prognostic tool for dentists.
Neutrophils are constant defenders of mucosal surfaces
While they are traditionally thought of as first responders during infection, it is not a trivial observation that neutrophils are constantly and constitutively recruited to mucosal sites throughout the body, where commensal, or “good” bacteria, are ever-present. This includes the gastrointestinal, respiratory and reproductive tracts, and importantly the oral cavity. The continuous recruitment of neutrophils to these tissue sites occurs in health, although in a pathological setting, such as periodontal disease, neutrophil recruitment is accelerated.
The oral cavity is a major interface with the external environment, with significant exposure to potential pathogens. Neutrophils, which are constantly recruited through the gingival lamina propria, across the junctional epithelium, and emerge into the gingival crevicular fluid and saliva, serve an important protective function in health.2,3The fundamental role of neutrophil recruitment to the oral cavity is underscored by our observation that, PMNs are sooner observed in the oral cavity than the blood in patients that have undergone hematopoietic stem cell transplants.4,5 It has been estimated that 50 to 250 million PMNs are recruited to the oral cavity each day in health. In chronic periodontal disease (PD) oral PMN load increases 4 to 10-fold.2,6
Oral mucosal surfaces are lined with a biofilm of commensal microbial organisms, and despite this being the natural state, and in fact necessary for health, oral neutrophils serve an important function by limiting the growth of these commensal microbes, including formation of a wall of neutrophils on the apical side of the junctional epithelium that separates and protects the oral surfaces from the gingival biofilm.7,8 Because oral biofilms are in constant flux due to constant environmental exposure of the mouth, neutrophils additionally function to limit the abundance of specific microbial strains in the oral ecosystem including keystone pathogens, and therefore help to maintain a homeostatic biofilm.
Neutrophils are important mediators of oral disease
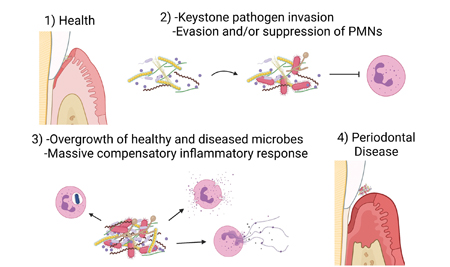
If and when oral PMNs fail to protect from and properly maintain the oral biofilm, a progressive dysbiotic state occurs, eventually triggering an augmented immune response. Excessive and chronic activation of oral neutrophils, a hallmark of periodontal disease, contributes to damage of periodontal connective tissue, loss of attachment, alveolar bone and tooth loss;9 yet despite the massive inflammatory response, the oral bacterial infection is not contained, and hyperactive neutrophils instead contribute to a worsening of the dysbiotic state. Compounds that reduce neutrophil activation or block the destructive mediators produced by neutrophils, such as reactive oxygen species, are potential therapeutic candidates for application in periodontal disease patients. Research suggests that, initially, neutrophil responses in the oral cavity are suppressed, as some pathogens have evolved mechanisms to subvert or evade neutrophils.10,11 (Fig. 1) The high levels of oral neutrophils that are characteristic of periodontal disease are a much later phenomenon that develops in the full blown disease. By tracking neutrophil activation and the oral inflammatory state in healthy individuals over time, it may be possible to identify a period of suppressed inflammation, the calm before the storm, that precedes the onset of periodontal disease.
This model for the mechanism of initiation of periodontal disease rests on the assumption that invading keystone pathogens that subvert or avoid neutrophil mediated destruction might also impact the ability of PMNs to respond normally to the commensal organisms that are also present in the oral cavity. Eventually this hindered response would result in increased bacterial load, feed-forward dysbiosis, tissue damage by way of bacterial virulence factors and tissue invasion by the dysbiotic oral bacteria, thereby triggering a massive compensatory inflammatory response.
Fig. 1: Oral neutrophil responses to microbial dysbiosis
Neutrophils as a diagnostic biomarker for oral disease
Strong evidence supports that sustained oral dysbiosis, associated with specific keystone pathogens, is coupled with the onset of periodontal disease.10,12,13 We3,14,15 and others16,17 have demonstrated that oral neutrophil counts are elevated in periodontitis patients, and that these neutrophils are in a hyper-inflammatory state, compared to neutrophils from the healthy oral cavity. We have developed flow cytometry based assays to measure various aspects of the oral neutrophil activation state, which we used to compare neutrophils from healthy controls and chronic periodontal disease patients.3 We demonstrated a pro-inflammatory phenotype associated with full blown chronic periodontal disease, while oral neutrophils in health displayed a partially activated (para-inflammatory) state. Oral neutrophils, which are readily available in saliva, thus constitute an important potential diagnostic biomarker for oral disease. Additionally, we have developed a model of ligature-induced periodontal disease using mice, including recovery and analysis of neutrophils from the mouse mouth, that show activation of the same oral neutrophils markers identified in humans.18 This mouse model represents a powerful research tool to test for novel therapeutic compounds for treatment of periodontitis.
Oral inflammation has long distance effects on neutrophils in the ciculation
In addition to localized tissue destructive effects in the mouth, oral inflammation can exhibit long distance effects that alter neutrophil activation in the circulation and bone marrow.19 In a recent study, we used mouse and human models of periodontal inflammation to analyze systemic/whole body immune effects.20 Using a mouse model of ligature-induced periodontal disease, we found that bone marrow neutrophil counts were elevated compared to healthy control mice. When these mice were exposed to an unrelated secondary inflammatory trigger, the presence of underlying periodontal inflammation caused a systemic neutrophil response that was previously not appreciated. Compared to mice that had peritonitis only, the mice with underlying perio had a greater increase in circulating neutrophil counts and greater neutrophil recruitment to the inflamed peritoneum. Interestingly, neutrophil recruitment to the colon, a completely independent site, was also increased in these ‘double-hit’ mice.20
Using a model of induced gingivitis we also found changes in blood neutrophils after the onset of oral inflammation in humans.20 Healthy volunteers were instructed to cease all oral hygiene practices for a three week period. During this period clinical features including bleeding on probing and gingival index were increased, and coincided with increased sensitivity of circulating neutrophils. Other groups have also identified increased neutrophils21 and pro-inflammatory cytokines16 in the circulation as a consequence of oral neutrophil recruitment. The evident far-reaching potential influence of oral health on systemic health further accentuates the importance of appropriate treatment of periodontal disease, through neutrophil-targeted and conventional treatment modalities.
Conclusion
Neutrophils are exquisitely sensitive to biochemical and physiological signals in their immediate environment, to which they respond on extremely short time-scales. Due to this volatile nature they have shown considerable experimental intractability. We have developed a method for fixing neutrophils in saliva that maintains their native state,3,22 and therefore facilitates high fidelity analysis of blood and oral neutrophils in a clinical setting. Due to the association of oral neutrophils with periodontal disease, and the ready availability of neutrophils in human saliva, these immune cells represent a significant opportunity for therapeutic intervention and as biomarkers in oral disease, whose potential has only just begun to be exploited.
Oral Health welcomes this original article.
References
- Nathan, C., Neutrophils and immunity: challenges and opportunities. Nature reviews.Immunology, 2006. 6(3): p. 173-182.
- Landzberg, M., et al., Quantifying oral inflammatory load: oral neutrophil counts in periodontal health and disease. J Periodontal Res, 2015. 50(3): p. 330-6.
- Fine, N., et al., Distinct Oral Neutrophil Subsets Define Health and Periodontal Disease States. J Dent Res, 2016. 95(8): p. 931-8.
- Cheretakis, C., Y. Dror, and M. Glogauer, A noninvasive oral rinse assay to monitor engraftment, neutrophil tissue delivery and susceptibility to infection following HSCT in pediatric patients. Bone Marrow Transplant, 2005. 36(3): p. 227-32.
- Cheretakis, C., et al., Timing of neutrophil tissue repopulation predicts restoration of innate immune protection in a murine bone marrow transplantation model. Blood, 2006. 108(8): p. 2821-6.
- Bender, J.S., H. Thang, and M. Glogauer, Novel rinse assay for the quantification of oral neutrophils and the monitoring of chronic periodontal disease. Journal of Periodontal Research, 2006. 41: p. 214-220.
- Ryder, M.I., Comparison of neutrophil functions in aggressive and chronic periodontitis. Periodontol 2000, 2010. 53: p. 124-37.
- Delima, A.J. and T.E. Van Dyke, Origin and function of the cellular components in gingival crevice fluid. Periodontol 2000, 2003. 31: p. 55-76.
- Irie, K., C.M. Novince, and R.P. Darveau, Impact of the Oral Commensal Flora on Alveolar Bone Homeostasis. Journal of Dental Research, 2014. 93(8): p. 801-806.
- Maekawa, T., et al., Porphyromonas gingivalis manipulates complement and TLR signaling to uncouple bacterial clearance from inflammation and promote dysbiosis. Cell Host Microbe, 2014. 15(6): p. 768-78.
- Oveisi, M., et al., Novel Assay To Characterize Neutrophil Responses to Oral Biofilms. Infect Immun, 2019. 87(2).
- Shurin, S.B., et al., A neutrophil disorder induced by capnocytophaga, a dental micro-organism. N Engl J Med, 1979. 301(16): p. 849-54.
- Darveau, R.P., et al., Local chemokine paralysis, a novel pathogenic mechanism for Porphyromonas gingivalis. Infect Immun, 1998. 66(4): p. 1660-5.
- Johnstone, A.M., et al., A hyperactive neutrophil phenotype in patients with refractory periodontitis. Journal of periodontology, 2007. 78(9): p. 1788-1794.
- Aboodi, G.M., et al., Salivary Cytoprotective Proteins in Inflammation and Resolution during Experimental Gingivitis – A Pilot Study. Front Cell Infect Microbiol, 2015. 5: p. 92.
- Figueredo, C.M., R.G. Fischer, and A. Gustafsson, Aberrant neutrophil reactions in periodontitis. J Periodontol, 2005. 76(6): p. 951-5.
- Cortes-Vieyra, R., C. Rosales, and E. Uribe-Querol, Neutrophil Functions in Periodontal Homeostasis. J Immunol Res, 2016. 2016: p. 1396106.
- Chadwick, J.W., et al., Tissue-specific murine neutrophil activation states in health and inflammation. J Leukoc Biol, 2021. 110(1): p. 187-195.
- Vitkov, L., et al., Connection between Periodontitis-Induced Low-Grade Endotoxemia and Systemic Diseases: Neutrophils as Protagonists and Targets. Int J Mol Sci, 2021. 22(9).
- Fine, N., et al., Periodontal Inflammation Primes the Systemic Innate Immune Response. J Dent Res, 2021. 100(3): p. 318-325.
- Christan, C., et al., White blood cell count in generalized aggressive periodontitis after non-surgical therapy. J Clin Periodontol, 2002. 29(3): p. 201-6.
- Fine, N., W. Khoury, and M. Glogauer, In Vitro Assay for Sensitive Determination of Human Blood PMN Responses. Methods Mol Biol, 2020. 2087: p. 235-241.
About the Author
 Noah Fine is a research associate in the Department of Dentistry at the University of Toronto. He is a specialist in neutrophil biology in the context of oral health and disease with 25 publications in the field. His research is focussed on identification of novel neutrophil subsets and activation states using cell surface biomarker identification by flow cytometry.
Noah Fine is a research associate in the Department of Dentistry at the University of Toronto. He is a specialist in neutrophil biology in the context of oral health and disease with 25 publications in the field. His research is focussed on identification of novel neutrophil subsets and activation states using cell surface biomarker identification by flow cytometry.
 Michael Glogauer is a Professor at the University of Toronto. His research and clinical interests focus on the role of the oral innate immune system in maintenance of health and the use of innate immune biomarkers to detect early stages of periodontal disease. Dr. Glogauer is Head of Dental Oncology, University Health Network – Princess Margaret Cancer Centre. He is a periodontist at OMGPerio.ca.
Michael Glogauer is a Professor at the University of Toronto. His research and clinical interests focus on the role of the oral innate immune system in maintenance of health and the use of innate immune biomarkers to detect early stages of periodontal disease. Dr. Glogauer is Head of Dental Oncology, University Health Network – Princess Margaret Cancer Centre. He is a periodontist at OMGPerio.ca.












