Editor’s note: Please click tables to see an enlarged version.
These are exciting times in the world of periodontology. The 2017 World Workshop, a combined collaboration by the European Federation of Periodontology (EFP) and the American Academy of Periodontology (AAP), has culminated in a new classification system for periodontal and peri-implant diseases and conditions. This is the first major update to the classification of periodontal disease since 1999 and is the most evidence-based and clinically relevant system that has ever been proposed. More than 170 leading clinicians and researchers from across the globe (including representation from Canadian periodontists) were involved in the monumental task of revising, clarifying and improving the classification system so we, the clinicians, can better communicate with each other and be more effective in our treatment of our patients.
Importantly, this classification system is the new standard of salient clinical information that all dental professionals around the world should be aware of and should adopt in their practice with regards to periodontal and peri-implant diseases.
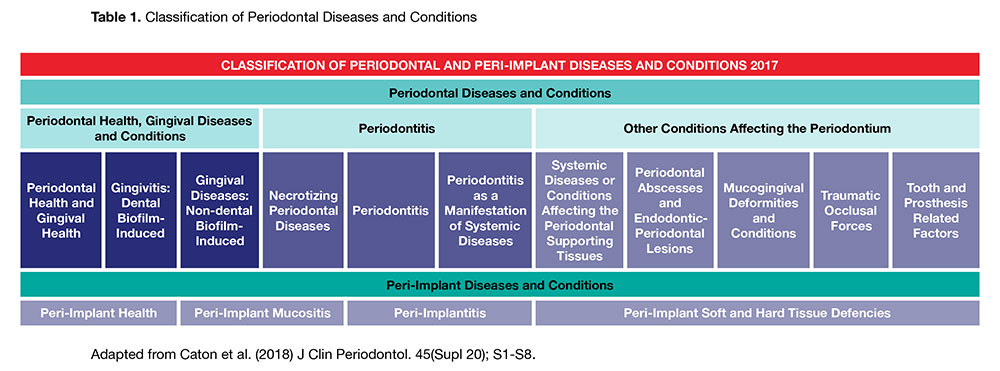
The comprehensive classification is based upon the most contemporary evidence and includes a staging and grading system for periodontitis, indicating severity and extent of disease, accounting for lifetime disease experience and considering the patient’s overall health status. The complete review of primary information and consensus reports for the innovative model for understanding and diagnosing periodontal diseases was simultaneously published in 17 articles including four review papers in the Journal of Clinical Periodontology and the Journal of Periodontology in June 2018. The new classification system (Table 1) will be presented for the first time in North America at the American Academy of Periodontology meeting in November 2018 in Vancouver.
This paper aims to distill the most striking changes and the most important concepts into several key tables suitable for immediate chair side implementation. There are many significant changes in this update that will improve the clinician’s understanding of periodontal disease progression, potential risk factors and allows the clinician to diagnose the patient based on a system of staging and grading, similar to the system used in the practice of oncology, never before employed in periodontal diagnosis.
Why Do We Need A New Classification System?
1. To facilitate an international language for clinical communication.
2. To enable proper diagnosis and prognostication for patient communication and education.
3. To ensure implementation of appropriate treatment.
4. To facilitate international population surveys of disease prevalence.
5. To enable research into the etiology, pathogenesis, natural history and treatment strategies.
Overview
The Classification System is presented in two parts: Part 1, Periodontal Diseases and Conditions and Part 2, Peri-Implant Diseases and Conditions. This executive summary focuses on Part 1, Periodontal Diseases and Conditions.
There are three subsections related to periodontal diseases (summarized in Table 1):
1. Periodontal Health, Gingival Diseases and Conditions
2. Periodontitis
3. Other Conditions affecting the Periodontium
What’s New?
Several unresolved issues and clarifications to the diagnosis and classification of periodontal disease and health have been established from the 2017 World Workshop. Firstly, a definition of periodontal health for patients with an intact or a reduced periodontium has been developed. This distinction was made to highlight the need for continued and comprehensive maintenance of patients after successful treatment of periodontitis. It is imperative to highlight that a patient with gingivitis can be treated and returned to a healthy state with low levels of gingival inflammation and disease, but that a patient with periodontitis is considered to be a periodontitis patient for life and requires life-long supportive care to prevent disease recurrence.1
In defining a state of health, gingivitis, or periodontal disease, it was agreed that bleeding on probing (BOP) should be among the primary parameters to set thresholds for diagnosis and treatment planning. Therefore, sites with BOP should be measured at every regularly scheduled interval to allow for the accurate assessment and measurement of periodontal health and timely prevention of progression towards a diseased state.
Secondly, a new identification and classification system of periodontitis has been defined. Updated from the 1999 classification of chronic, aggressive (localized or generalized), necrotizing or as a manifestation of systemic disease, the newly revised classification system is based on a staging and grading system. Also updated, the previous four subsets of periodontitis have been simplified into three: necrotizing periodontitis, periodontitis as a manifestation of systemic disease, and periodontitis (previously considered as either chronic or aggressive).
All updates to the classifications for periodontal and peri-implant diseases from the 1999 classifications can be found in the summary statement prepared by the 2017 World Workshop2.
Periodontal Health, Gingival Diseases and Conditions
Defining a state of periodontal health is essential to creating a common reference point for the assessment and evaluation of treatment in periodontal disease and gingivitis.
Clinical periodontal health is clearly distinct from pristine clinical health (Table 2). 3 Pristine clinical health is rare, but a realistic entity. It is defined by no attachment loss, no bleeding upon probing, and no anatomical loss of periodontal structures. There should be no signs of inflammation which include redness, clinical swelling, edema and pain. However, much like antivirus software on a computer, polymorphonuclear leukocyte surveillance is always on and active, which is a very important physiological and not a pathological process. As such, even if a patient has prolonged clinically healthy gingiva, it is always histologically characterized by a small inflammatory infiltrate. Therefore, pristine conditions are rare.
Rather, clinical periodontal health should be defined as a state free from inflammatory periodontal disease or gingivitis that allows an individual to function normally and avoid physical or mental consequences due to current or past disease. Furthermore, a consideration should be made for patients with an intact (without attachment loss or radiographic bone loss) or reduced periodontium (with attachment loss and decreased alveolar height). In a reduced periodontium, periodontal disease stability involves successful treatment of periodontal disease resulting in minimal BOP, improvements in periodontal probing depth (PPD) and attachment levels as well as a lack of progressive destruction.
Periodontal disease remission and control is a reasonable treatment outcome for individuals with controllable modifying factors (i.e., obesity, diabetes and smoking). Remission/control is the period throughout the course of the disease during which treatment has resulted in reduction (but not total resolution) of inflammation and some improvements in periodontal probing depths and attachment levels but not optimal control of local or systemic contributing factors.
Gingival diseases can be separated into and defined as dental biofilm-induced or non-dental biofilm-induced. Biofilm-induced gingivitis is a site-specific inflammatory condition initiated by dental biofilm accumulation and characterized by gingival redness, edema and the absence of periodontal attachment loss. The key clinical features of plaque-induced gingivitis include erythema, edema, bleeding, tenderness, heat, loss of function and gingival enlargement. It is commonly painless and therefore, most patients are unaware of the disease or unable to recognize it. Patients may however often report bleeding gums, pain (soreness), halitosis, difficulty eating, or red swollen gums. Unlike periodontitis, gingivitis is completely reversible with the mechanical removal of dental biofilm. A summary of parameters defining a healthy state compared to two diseased states is presented in Table 3. 4
Defining a ‘site’ of gingivitis is much different than defining a ‘case’ of gingivitis. While clinical gingival inflammation is well-characterized and easily quantifiable on a site-specific basis, a ‘case’ of gingivitis is meant to define the disease on a whole-patient level. 4
The diagnosis of dental biofilm-induced gingivitis is graded and identified based on the extent and the severity of a patient’s BOP score (%). When only a few sites (<10%) are affected by gingival inflammation (mild redness and/or a broken line of bleeding rather than edema or an immediate unbroken line of bleeding on probing), this is defined as incipient gingivitis. 5 This newly defined term, whereby only a few sites are affected, may even be regarded as within the spectrum of clinical periodontal health. Incipient gingivitis may rapidly progress to localized gingivitis (10-30% BOP) if left untreated. Generalized gingivitis involves BOP scores of greater than 30%.
Similarly, the severity of gingival inflammation can be categorized as mild, moderate or severe:
- Mild gingival inflammation: area with a minor change in the colour and little change in texture of the tissue.
- Moderate gingival inflammation: area with glazing, redness, edema, enlargement and BOP.
- Severe gingival inflammation: area of overt redness and edema with a tendency toward bleeding when touched rather than probed.
The extent and severity of gingival enlargements in the diagnosis of gingivitis have also been updated in the 2017 classification system. Most often due to medication, gingival enlargements may either be localized (single tooth or a single group of teeth) or generalized (apparent throughout the whole mouth). Mild gingival enlargement involves the gingival papilla only, whereas moderate gingival enlargement involves the gingival papilla and the marginal gingiva. Severe gingival enlargement involves the gingival papilla, marginal gingiva, and the attached gingiva. 5
Several local factors are known to exacerbate dental biofilm-induced gingivitis including prominent subgingival restorations and hyposalivation. Xerostomia, a symptom caused by perceived lack of salivation (not a diagnosis) may make plaque control difficult and gingival inflammation may be worsened. Similarly, medications which cause dry mouth such as antihistamines, decongestants, antidepressants, and antihypertensives (among others), can cause dental caries, taste disorders, halitosis, and inflammation of the oral mucosa, tongue and gingiva.
Updated from the 1999 classification system, oral contraceptives and menstrual cycle have been removed as a modifying risk factor in the new 2017 classification system. It was previously believed that oral contraceptives and hormonal changes associated with the menstrual cycle were associated with gingival inflammation, gingival enlargement, and increases in gingival crevicular fluid production. More recent literature disproves these assumptions. 5
Several systemic risk factors impact dental biofilm-induced gingivitis including uncontrolled hyperglycaemia (primarily in individuals with type I diabetes mellitus), leukemia, smoking, and malnutrition (i.e., Vitamin C deficiency). 5 In smokers, plaque accumulation and disease progression is exacerbated, however, smokers experience fewer clinical signs and symptoms of gingival inflammation, often masking the disease to dental health professionals. Additional complexity to the diagnosis of gingivitis comes from the large variations in the extent and severity of gingivitis in individuals with these systemic risk factors. These dramatic differences in the severity and extent of gingivitis among individuals may be due to modifying risk factors such as medications, the tooth and/or root anatomy, restorative factors, or endodontic factors all compounding in the micro-environment of the oral cavity.
Non-dental biofilm-induced gingival diseases are less common but are often of major significance for patients. These are often manifestations of systemic conditions. They may represent pathologic changes limited to the gingiva and the classification is based on the etiology of the lesions (Table 4). Non-plaque-induced gingival diseases and conditions are usually not resolved by mechanical plaque removal. 1 These diseases and conditions can be classified into eight general categories; genetic/developmental disorders, specific infections, inflammatory or immune conditions and lesions, reactive processes, neoplasms, endocrine/nutritional/metabolic disease, traumatic lesions, or gingival pigmentation. A detailed description of the clinical presentation, etiology, and associated conditions of each of these non-plaque-induced gingival diseases has been elegantly summarized by Holmstrup and colleagues. 6
Regardless of the cause, extent or severity of gingival disease, the treatment of gingivitis remains unchanged. Clear and concise oral hygiene instruction and increased patient motivation for adequate home care is imperative in the treatment of gingivitis. Professional mechanical plaque removal which may be supplemented by adjunctive use of antimicrobial/anti-inflammatory oral care products is often recommended. When diet and smoking are modifiable risk factors for your patients, dietary advice and tobacco counselling are strongly recommended. The recommendations for treatment of gingivitis remains unchanged in the new and current classifications.
Periodontitis
Periodontitis is defined as a chronic multifactorial inflammatory disease associated with bacterial dysbiosis and characterized by progressive destruction of the tooth supporting structures. The primary features of the disease include the loss of periodontal tissue support manifested through clinical attachment loss (CAL) and radiographic bone loss, presence of periodontal pocketing and gingival bleeding. This is evidenced as CAL by circumferential assessment of the erupted dentition with a standardized periodontal probe with reference to the cemento-enamel junction (CEJ).
Periodontitis is a complex and dynamic interplay of multiple causal factors including lifestyle, tooth anatomy, systemic diseases, genetics, and environment. Prior to the newly developed system, the classification of periodontal disease was very broad, encompassing several categories of disease. As an update from the 2017 World Workshop, the new classification system reduces to only three categories by collapsing aggressive and chronic periodontitis into a single category as the pathophysiology was considered to be too similar. 7
Based on the pathophysiology, three categories of periodontitis have been defined:
1. Necrotizing periodontitis
2. Periodontitis as a direct manifestation of systemic disease
3. Periodontitis
Regardless of the category, in the clinical context a patient is considered a periodontitis case if (1) interproximal CAL is detectable at two or more non-adjacent teeth and when (2) buccal or oral CAL ≥3mm with pocketing ≥3mm is detectable at two or more teeth, but the observed CAL cannot be due to non-periodontitis related causes such as: 8
a. Gingival recession of traumatic origin,
b. Dental caries extending in the cervical area of the tooth,
c. Presence of CAL on the distal aspect of a second molar and associated with malposition or extraction of a third molar,
d. Endodontic lesion draining through the marginal periodontium, and
e. Occurrence of a vertical root fracture.
Given the measurement error when quantifying CAL with a standard periodontal probe, the newly proposed clinical definition does not stipulate a specific threshold of CAL to avoid any misclassification of early periodontitis cases as gingivitis. While periodontal inflammation (generally measured as BOP) is an important clinical parameter, the presence of BOP does not change the classification or diagnosis of periodontitis and its severity. 8
Necrotizing periodontitis is an inflammatory process characterized by a prominent bacterial invasion and ulceration of the epithelium. Necrotizing periodontitis has a distinct pathophysiology characterized by the presence of necrosis of the interdental papillae, gingival bleeding, halitosis, pain, and rapid bone loss. Necrotizing periodontal diseases and conditions are strongly associated with severe impairment of the host immune system. Some pre-disposing conditions include HIV/AIDS, immunosuppressed patients, severe malnourishment, or viral infections.
Periodontitis as a manifestation of systemic diseases considers the multifactorial etiology of the disease and the level of complexity of the risk and management of the disease. The category of periodontitis as a manifestation of systemic diseases accounts for more than one third of the classified cases of periodontitis. These systemic diseases have a major impact on the loss of periodontal tissue through its influence on periodontal inflammation.
Systemic disorders can impact periodontal inflammation via an immunological response (i.e., leukocyte adhesion deficiency syndromes), by affecting the oral mucosa, gingival tissue (i.e., epidermolysis bullosa), connective tissues (i.e., systemic lupus, Ehlers-Danos syndrome), or through metabolic or endocrine disorders (i.e., obesity, osteoporosis, diabetes mellitus, hypophosphotasia, glycogen storage diseases). Similarly, systemic inflammatory diseases such as rheumatoid arthritis or inflammatory bowel disease can impact periodontal health and influence the pathogenesis of periodontitis. 9
Although it has long been known that oral health is inextricably linked to overall health, today more than ever, the linkages between periodontics and medicine are stronger and even more well defined. The collaboration required between dental health professionals and physicians has never been more necessary to provide our mutual patients with the most current multi-disciplinary treatment and care.
A differential diagnosis of the category of periodontitis is based on the history and the specific presentation of necrotizing periodontitis or the presence or absence of an uncommon systemic disease that alters the immune response. All of the remaining clinical cases of periodontitis which meet the above criteria should be diagnosed as periodontitis and then further characterized using a staging and grading system. This newly developed staging and grading system (similar to that used in the practice of oncology) shown in Table 5, describes clinical presentation, prognosis, and treatment planning. 8
Stage (1-4) is largely dependent on the severity and the anticipated complexity of the management of the disease (Table 5). 8 Key elements in the staging of periodontitis involve classifying:
1. Degree of periodontal breakdown,
2. Number and distribution of teeth with detectable breakdown,
3. Direct or indirect evidence of the rate of the destruction of the periodontal tissues,
4. Complexity of management (i.e., type of bone loss (horizontal or angular), probing depths, furcation involvement, tooth mobility, number of missing teeth, occlusal and functional aspects),
5. Causal factors (i.e., systemic health, lifestyle factors, genetics, environmental factors, tooth anatomy, etc.).
Grade (A-C) encompasses further information about biological features including the rate of progression, assessment of the risk of further progression, an analysis of possible poor outcomes of treatment, and an assessment of the risk of the disease to negatively impact the general health of the patient (Table 6). 8 For a full description of the phenotypes of each stage and grade of periodontitis, please refer to primary research article written by Tonetti and colleagues. 8
This newly developed framework provides the clinician the ability to define each individual case by a simple matrix of Stage (severity and complexity) and Grade (evidence or risk of progression). A step-by-step guide for staging and grading a patient is described in Table 7.
Other Conditions Affecting The Periodontium
Different still from the relatively slow progression of periodontitis are periodontal abscesses, lesions from necrotizing periodontal diseases and endo-periodontal lesions, all of which affect the periodontium. These conditions differ from periodontitis in their rapid onset and destruction of periodontal tissues, infection, and the pain and discomfort that they cause patients, often leading them to seek emergency care.
Periodontal abscesses represent approximately 8-14% of all dental emergencies. The most prominent sign of a periodontal abscess is an ovoid elevation in the gingiva along the lateral part of the root.10 Bleeding and suppuration on probing, presence of a deep periodontal pocket, increased tooth mobility, and bone loss are also commonly observed symptoms during the oral examination. In periodontitis patients, a periodontal abscess may occur as a result of disease exacerbation that is supported by the existence of deep pockets, the presence of furcation involvement, or a vertical defect. Periodontal abscess may also occur in previously healthy sites (i.e., patients without periodontitis) because of harmful habits (i.e., nail biting, clenching, etc.), impaction of foreign bodies, orthodontic factors, gingival overgrowth, or alteration of the root surface (i.e., cemental tears, enamel pearls, perforations, external root resorption, perforations, cracked tooth syndrome, etc.).10
Regardless of the cause of periodontal abscesses, they may lead to tooth loss and may even cause systemic infections, highlighting the importance of quick diagnosis and immediate treatment for the patient. 10
Endodontic-periodontal lesions are a further classified clinical condition involving both the periodontal tissue and the pulp. The most common signs and symptoms of an endo-perio lesion are deep periodontal pockets reaching (or nearing) the apex of the tooth and a negative or altered response to pulp vitality tests. 10 Endo-perio lesions can be associated with either endodontic or periodontal infections or associated with trauma and iatrogenic factors (i.e., root perforation, fracture, or external root resorption) often causing root damage. The first steps in the diagnosis of an endo-perio lesion should be to assess the patient’s history and clinical or radiographic examination to assess the integrity of the root. Further, a periodontal assessment, including probing depths should be conducted to assess the prognosis for the tooth. A tooth with an endodontic-periodontal lesion can be categorized as having a hopeless, poor, or favourable prognosis. 10
Mucogingival defects including gingival recession occur in 88% of adults aged ≥65 years and 50% of people aged 18-64 years. 11 Gingival recession, defined as an apical shift of the gingival margin with respect to the CEJ, is associated with CAL and exposure of the root surface to the oral environment. Gingival recession is frequently associated with dentinal hypersensitivity, impaired esthetics, and carious and non-carious cervical lesions.
Suggested by the 2017 World Workshop, the mucogingival condition should be described by the periodontal phenotype. This is determined by the gingival thickness, keratinized tissue width and bone morphotype. The periodontal phenotype indicates the appearance of the gingiva that may change through time depending on tooth position, mechanical factors (i.e., improper tooth brushing habits), orthodontics, and even genetic traits. 12
When considered together, gingival thickness and keratinized tissue width can determine the gingival biotype of a patient. Three biotypes exist:
1. Thin scalloped biotype: slender triangular crown, subtle cervical convexity, interproximal contacts close to the incisal edge and a narrow zone of keratinized tissue, clear thin delicate gingiva, and a relatively thin alveolar bone.
2. Thick flat biotype: square shaped tooth crowns, pronounced cervical convexity, large interproximal contact located more apically, a broad zone of keratinized tissue, thick fibrotic gingiva, and a comparatively thick alveolar bone.
3. Thick scalloped biotype: thick fibrotic gingiva, slender teeth, narrow zone of keratinized tissue, and a pronounced gingival scalloping.
To assess gingival thickness, a periodontal probe should be used. After being inserted into the sulcus, if the probe can be seen shining through the gingival tissue it can be defined as thin (≤1.0mm). If it is not visible, the gingival tissue can be defined as thick (>1.0mm).
Further diagnostic consideration of the recession depth and interdental CAL should also be considered in cases of mucogingival defects. A new classification of gingival recession with reference to interdental CAL has been proposed, replacing the Miller classification: 13
- Recession Type 1 (RT1): Gingival recession with no loss of interproximal attachment. Interproximal CEJ is clinically not detectable at both mesial and distal aspects of tooth.
- Recession Type 2 (RT2): Gingival recession associated with loss of interproximal attachment. The amount of interproximal attachment loss is less than or equal to the buccal attachment loss.
- Recession Type 3 (RT3): Gingival recession associated with loss of interproximal attachment. The amount of interproximal attachment loss is higher than the buccal attachment loss.
Based on periodontal phenotype, gingival recession and root surface conditions, a new classification and treatment considerations have been put forth by the 2017 World Workshop.
In the absence of gingival recession:
1. Thick gingival biotype – prevention through good oral hygiene and monitoring.
2. Thin gingival biotype – attention and careful monitoring by the clinician. With respect to cases with severe thin gingival biotype, mucogingival surgery should be considered to prevent future mucogingival damage. This applies especially in cases in which additional orthodontics, restorative dentistry, or implant therapy are planned.
In the presence of gingival recession:
A treatment-orientated approach based on the interdental CAL measurement and recession depth, periodontal phenotype, root surface condition, tooth position, cervical tooth wear and number of adjacent recessions should always be considered.
1. Conservative clinical approach – charting and monitoring periodontal and root surface lesions.
2. Thin biotypes and when motivated by patient concern – mucogingival surgery for root coverage and CEJ reconstruction when needed. This applies especially in cases in which additional orthodontics, restorative dentistry, or implant therapy are planned.
It is important to note that any amount of gingiva is sufficient to maintain periodontal health when optimal oral hygiene is obtained. However, if existing gingival recession is left untreated, it is highly likely that recession depths will increase over time. 14 Limited evidence suggests that gingival recession does not impair the long-term survival of teeth, however, it may promote dentin hypersensitivity, and compromise esthetics.
Traumatic occlusal forces and their role in the initiation and progression of periodontitis remains one of the most controversial and contentious subjects in the field of periodontology. Any occlusal force resulting in injury to the tooth or the periodontal attachment may be indicated by fremitus, thermal sensitivity, tooth mobility, excessive occlusal wear, tooth migration, discomfort or pain during mastication, fractured teeth, radiographically widened periodontal ligament space, root resorption, or cemental tear. 15 A review of previous studies generally conclude that traumatic occlusal forces do not initiate or accelerate the process of periodontitis or connective tissue attachment loss and that with good plaque control, orthodontic forces do not have adverse effects on the periodontium. 15 Similarly, existence of abfraction and its implication on gingival recession or non-carious cervical lesions as a result of traumatic occlusal forces are not supported in the current literature. 15 However, a reduction of tooth mobility may improve periodontal treatment outcomes. 16
The fabrication and presence of dental prostheses and tooth-related factors have an impact on periodontal health and disease state. Within this subcategory of factors influencing the periodontium, the most notable change is the replacement of the dated term, biological width, with supracrestal tissue attachment. 17 An impingement of the supracrestal tissue attachment is associated with inflammation and a loss of periodontal supporting tissue. This underscores the importance of optimal restoration margins that are located within the gingival sulcus. If the supracrestal tissue attachment is respected and patients are compliant with home care instructions and undergo regular professional periodontal maintenance, gingival inflammation and its potential for progression to periodontitis can be prevented.
Tooth anatomical factors such as root abnormalities, fractures and tooth relationships within the dental arch (root proximity) and with opposing dentition can enhance plaque retention. 17 Other localized anatomical tooth factors such as cervical enamel projections, enamel pearls and developmental grooves can modify or predispose patients to plaque induced gingival diseases and periodontitis. Tooth-supported or retained restorations (either fixed or removable) must be optimally designed with precision and fabricated with appropriate materials as hypersensitivity reactions can occur in some patients in response to dental materials. Sub-optimal design and manufacture of dental prostheses can be associated with plaque retention and the eventual loss of supporting periodontal tissues. Restoration margins located within the gingival sulcus are not considered to be a cause of gingivitis and inflammation as long as the patient is compliant with their self-performed plaque control. 17
Conclusions
In conclusion, this new classification system presents a unique opportunity to escalate our precision care for our patients, spark conversations with our medical colleagues about treating the whole patient and push us towards exploring exciting research questions. In this volatile, uncertain, complex and ambiguous world of today, adapting and learning are fundamental components to our success and continued relevance.
For the complete suite of reviews, case definition papers, and consensus reports, please visit perio.org/2017wwdc. OH
Oral Health welcomes this original article.
References
- Chapple ILC, Mealey BL, Van Dyke TE, Bartold PM, Dommisch H, Eickholz P, Geisinger ML, Genco RJ, Glogauer M, Goldstein M, et al. (2018) Periodontal health and gingival diseases and conditions on an intact and a reduced periodontium: Consensus report of workgroup 1 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J Clin Periodontol. 45(Suppl 20): S68-S77.
- Caton JG, Armitage G, Berglundh T, Chapple ILC, Jepsen S, Kornman KS, Lealey BL, Papapanou PN, Sanz M, Tonetti MS. (2018) A new classification scheme for periodontal and peri-implant diseases and conditions–Introduction and key changes from the 1999 classification. J Clin Periodontol. 45(Suppl 20): S1-S8.
- Lang NP and Bartold PM. (2018) Periodontal Health. J Clin Periodontol. 45(Suppl 20): S9-S16.
- Trombelli L, Farina R, Silva CO, Tatakis DN. (2018) Plaque-induced gingivitis: Case definition and diagnostic considerations. J Clin Periodontol. 45(Suppl 20): S44-S67.
- Murakami S, Mealey BL, Mariotti A, Chapple ILC. (2018) Dental plaque-induced gingival conditions. J Clin Periodontol. 45(Suppl 20): S17-S27.
- Holmstrup P, Plemons J, Meyle J. (2018) Non-plaque-induced gingival diseases. J Clin Periodontol. 45(Suppl 20): S28-S43.
- Fine DH, Patil AG, Loos BG. (2018) Classification and diagnosis of aggressive periodontitis. J Clin Periodontol. 45(Suppl 20): S95-S111.
- Tonetti MS, Greenwell H, Kornman KS. (2018) Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. J Clin Periodontol. 45(Suppl 20): S149-S161.
- Albandar JM, Susin C, Hughes FJ. (2018) Manifestations of systemic diseases and conditions that affect the periodontal attachment apparatus: case definitions and diagnostic considerations. J Clin Periodontol. 45(Suppl 20): S171-S189.
- Herrera D, Retamal-Valdes B, Alonso B, Feres M. (2018) Acute periodontal lesions (periodontal abscesses and necrotizing periodontal diseases) and endo-periodontal lesions. J Clin Periodontol. 45(Suppl 20): S78-S94.
- Kassab MM and Cohen RE. (2003) The etiology and prevalence of gingival recession. J Am Dent Assoc. 134: 220-225.
- Cortellini P and Bissada NF. (2018) Mucogingival conditions in the natural dentition: Narrative review, case definitions, and diagnostic considerations. J Clin Periodontol. 45(Suppl 20): S190-198.
- Cairo F, Nieri M, Cincinelli S, Mervelt J, Pagliaro U. (2011) The interproximal CAL to classify gingival recessions and predict root coverage outcomes: An explorative and reliability study. J Clin Periodontol. 38:661-666.
- Chambrone L and Tatakis DL. (2016) Long-term outcomes of untreated buccal gingival recessions. A systematic review and meta-analysis. J Periodontol. 87: 1371-1378.
- Fan J and Caton JG. (2018) Occlusal trauma and excessive occlusal forces: Narrative review, case definitions, and diagnostic considerations. J Clin Periodontol. 45(Suppl 20): S199-S206.
- Cortellini P, Tonetti MS, Lang NP, Suvan JE, Zucchelli G, Vangsted T, Silvestri M, Rossi R, McClain P, Fonzar A, et al. (2001) The simplified papilla preservation flap in the regenerative treatment of deep intrabony defects: Clinical outcomes and postoperative morbidity. J Periodontol. 72: 1702-1712.
- Ercoli C and Caton JG. (2018) Dental prostheses and tooth-related factors. J Clin Periodontol. 45(Suppl 20): S207-S218.
About the Authors
 Dr. Fritz is a full-time periodontist in Fonthill, ON and is on a mission to redefine the way people think about periodontal and implant wellness. He leads an extraordinary, collaborative, empowered t eam of clinicians, makers, scientists and artists who are all working together to innovate the dental specialty of periodontics and redefine the patient experience.
Dr. Fritz is a full-time periodontist in Fonthill, ON and is on a mission to redefine the way people think about periodontal and implant wellness. He leads an extraordinary, collaborative, empowered t eam of clinicians, makers, scientists and artists who are all working together to innovate the dental specialty of periodontics and redefine the patient experience.

Wendy Ward is a Professor and Canada Research Chair in the Department of Kinesiology in the Faculty of Applied Health Sciences at Brock University. Her research program investigates how early diet sets a trajectory for a stronger, healthier skeleton at adulthood, and also how diet can support bone health at older life stages. Within this research program, a number of novel foods and food components are studied: vitamin D, soy and its isoflavones, omega-3 fatty acids in flaxseed and fish oil, and tea and its flavonoids.

Amanda Longo is the Chief Innovation Officer and Director of Strategy of a world-class periodontal practice in Fonthill, ON. Her role involves strategic innovation of the practice and of the profession itself to ensure the specialty of periodontology is positioned for the patient of today and of tomorrow. Dr. Longo’s doctoral thesis examined the use and safety of micro-computed tomography as a method to quantify the microarchitecture of bone. She investigated the effect of intermittent radiation exposure throughout the lifespan on the quality of bone health.