Dentin hypersensitivity (DH) is a clinical symptom experienced due to exposed dentin from either enamel loss (erosion, abrasion, abfraction) or removal of cementum exposed through gingival recession. DH is seen commonly in patients with gingival recession, dental erosion, toothbrush abrasion, or following dental bleaching. Aside from the discomfort experienced from eating, drinking, or exposure to cold air, DH may also prevent patients from maintaining proper home oral hygiene or create discomfort during professional dental care. To diagnose a patient with DH, several other clinical diagnoses should be ruled out, including caries, cracked tooth, gingival inflammation, pulpitis, and post-restorative sensitivity.1
A survey of dentists and hygienists reported that the most common treatments recommended for DH include application of a desensitizer (41%), recommendation of a sensitivity toothpaste (39%) and modification of habits (15%, stop drinking cold water, modify brushing technique and drink through a straw).2 This review will focus on the methods of treatment of DH as well as the use of desensitizers to prevent post-operative sensitivity following restorative procedures.
Mechanism of action of different desensitizing agents
Multiple theories have been proposed to demonstrate the reasons for dentinal hypersensitivity, but the most accepted one is the hydrodynamic theory. According to this theory, dentin hypersensitivity is caused by the disturbance of fluid movement inside the tubules due to pressure changes, which subsequently irritate the nerve endings in the pulp.3 Clinical studies have shown teeth with DH to have dentin tubules patent from the oral environment to the pulp, as many as eight times more patent tubules than non-sensitive teeth, and tubules twice as wide as those in non-sensitive teeth.4 Therefore, hypersensitivity is treated by either blocking the exposed tubules and inhibiting fluid flow across the dentinal tubules or decreasing the excitability of the intradental nerve fibers.
Agents that are used to occlude dentinal tubules include those containing glutaraldehyde, oxalate salts, calcium, fluoride, or light cured resins. These agents work to occlude dentinal tubules by coagulating proteins, precipitating crystals, or polymerizing resin. Alternatively, potassium salts act by diffusion along the dentinal tubules and decreasing the excitability of the intradental nerve fibers by blocking the axonic action.5
Sensitivity toothpastes
Sensitivity toothpastes contain either agents to decrease nerve excitability, such as potassium salts (potassium nitrate, potassium chloride, or potassium citrate) or agents to occlude dentinal tubules (stannous fluoride, arginine, nanohydroxyapatite, amorphous calcium silicate, strontium, calcium sodium phosphosilicate). A systematic review and meta-analysis of clinical trials reported that all the forementioned active ingredients reduced sensitivity more than a placebo control other than strontium and calcium sodium phosphosilicate.6
In-office desensitizers
Desensitizers are agents applied directly to the exposed dentin in the dental office. There are several different types of dentin desensitizers, including those which contain glutaraldehyde, oxalate salts, calcium phosphates, fluoride, or light-cured resins.
Glutaraldehyde based desensitizers are often a combination of 5% glutaraldehyde and 35% hydroxyethylmethacrylate (HEMA). Examples of these desensitizers include GLUMA (Heraeus), Microprime-G (Danville), G5 All-purpose desensitizer (Clinician’s Choice), and Telio CS Desensitizer (Ivoclar Vivadent). These desensitizers work by reacting with serum albumin in dentinal fluid, which both induces the precipitation of the serum albumin as well as the polymerization of the HEMA.7 Gluteraldehyde can cause soft tissue necrosis and therefore should be rinsed off following application.8 Rubber dam isolation may be another option for soft tissue protection.
Oxalate desensitizers work by reacting with hydroxyapatite in dentinal tubules and forming calcium oxalate crystals to occlude dentinal tubules. Examples of oxalate desensitizers include D’Sensitize (Dental Savings Club). Tubule occlusion with oxalate desensitizers has been shown to be effective even after acid challenge.9
Calcium phosphate containing desensitizers work by the creation of hydroxyapatite within dentinal tubules.10 Calcium phosphate desensitizers include varnishes with amorphous calcium phosphate (Enamel Pro Varnish, Premier), tri-calcium phosphate (Clinpro White varnish, 3M; FluoroCal, Bisco), casein phosphopeptide stabilized amorphous calcium phosphate (MI Varnish, GC), and Xylitol coated calcium and phosphate (Embrace Varnish, Pulpdent). Calcium phosphate desensitizers can also be placed in prophy pastes, such as NuPro (Dentsply) and EnamelPro (Premier). Additionally, a powder liquid desensitizer paste composed of calcium tetra phosphate and anhydrous dicalcium phosphate is available (Teethmate Desensitizer, Kuraray Noritake). Calcium phosphate containing varnishes, prophy pastes and pastes have all been shown to cause occlusion of dentin tubules and reduced dentin permeability, however, the results were significantly diminished following erosion/abrasion cycling.11
In-office fluoride-containing products (gels, foams, varnish, prophy pastes) may be used as desensitizers as fluoride may halt dentinal fluid movement through precipitation of CaF2 and fluorapatite in dentinal tubules.12 Additionally, silver diamine fluoride may be used to treat sensitivity as it is FDA approved for this use.
Dental adhesives (also called dentin bonding agents) are light-cured resins that can be used as desensitizers by depositing resin tags and covering exposed dentinal tubules. However, dentin bonding agents are hydrophobic agents that should be placed in a dry field.
Another option for desensitization is the use of a dental laser. Nd:YAG,Er, Cr:YSGG, and CO2 lasers have all been shown to melt peritubular dentin which can partially or totally occlude dentinal tubules.13
A laboratory study compared the use of glutaraldehyde, oxalate, fluoride and light-cured resin desensitizers and determined that the oxalate and light-cured resin adhesives had the greatest effect on reducing dentinal fluid flow.14 On the other hand, a review of clinical trials comparing the efficacy of desensitizers reported that glutaraldehyde-containing desensitizers and laser treatment had the greatest effect on reducing DH.15
Desensitizers used for post-operative sensitivity
Several aspects of the bonding procedure have been credited for causing post-operative sensitivity in resin composites, including over-etching dentin, insufficient infiltration of adhesive into the hybrid layer, insufficient polymerization of the adhesive, enzymatic or hydrolytic degradation of the hybrid layer over time, and polymerization shrinkage leading to microfractures of the tooth or microleakage at the tooth-restoration interface. Interestingly, clinical trials have reported no difference in post-operative sensitivity with the use of self-etch vs. etch-and-rinse adhesives,16 bonding to wet vs. dry dentin17 or the placement of bulk-fill vs. layered composites.18 There is low level evidence that the placement of a glass ionomer or calcium hydroxide liner may reduce post-operative sensitivity with resin composites.19
The use of desensitizers prior to placement of adhesive in bonded composite restorations has been proposed. Placement of glutaraldehyde-containing agents may help preserve the dentin bond by strengthening collagen network prior to application of an adhesive.20
Unfortunately, clinical trials with the use of glutaraldehyde and oxalate desensitizers prior to application of an adhesive have shown no improvement in post-operative sensitivity.21-24
Summary
The use of sensitivity toothpastes or in-office desensitizers may help improve DH. A clinical case is presented in Figs. 1-3 in which a patient suffering with DH following post-orthodontic gingival recession was treated with an in-office desensitizer.
Fig. 1
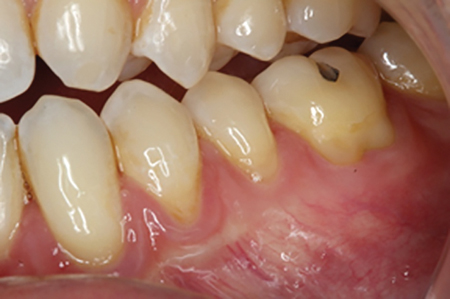
Fig. 2A

Fig. 2B
Fig. 3A

Fig. 3B

Oral Health welcomes this original article.
References
- Canadian Advisory Board on Dentin Hypersensitivity. Consensus-based recommendations for the diagnosis and management of dentin hypersensitivity. J Can Dent Assoc. 2003 Apr;69(4):221-6. PMID: 12662460.
- Clark D, Levin L. Tooth hypersensitivity treatment trends among dental professionals. Quintessence Int. 2018;49(2):147-151. doi: 10.3290/j.qi.a39510. PMID: 29234741.
- West NX, Lussi A, Seong J, Hellwig E. Dentin hypersensitivity: pain mechanisms and aetiology of exposed cervical dentin. Clin Oral Investig. 2013 Mar;17 Suppl 1:S9-19. doi: 10.1007/s00784-012-0887-x. Epub 2012 Dec 9. PMID: 23224116.
- Absi EG, Addy M, Adams D. Dentine hypersensitivity: a study of the patency of dentinal tubules in sensitive and non-sensitive cervical dentine. Journal of clinical periodontology. 1987 May;14(5):280-4.
- Markowitz K, Kim S. The role of selected cations in the desensitization of intradental nerves. Proc Finn Dent Soc. 1992;88 Suppl 1:39-54. PMID: 1508896.
- Bae JH, Kim YK, Myung SK. Desensitizing toothpaste versus placebo for dentin hypersensitivity: a systematic review and meta-analysis. J Clin Periodontol. 2015 Feb;42(2):131-41. doi: 10.1111/jcpe.12347. Epub 2015 Jan 9. PMID: 25483802.
- Qin C, Xu J, Zhang Y. Spectroscopic investigation of the function of aqueous 2-hydroxyethylmethacrylate/glutaraldehyde solution as a dentin desensitizer. Eur J Oral Sci. 2006 Aug;114(4):354-9. doi: 10.1111/j.1600-0722.2006.00382.x. PMID: 16911108.
- Arenholt-Bindslev D, Hörsted-Bindslev P, Philipsen HP. Toxic effects of two dental materials on human buccal epithelium in vitro and monkey buccal mucosa in vivo. Scand J Dent Res. 1987 Dec;95(6):467-74. doi: 10.1111/j.1600-0722.1987.tb01962.x. PMID: 3122307.
- Pereira JC, Segala AD, Gillam DG. Effect of desensitizing agents on the hydraulic conductance of human dentin subjected to different surface pre-treatments—an in vitro study. Dent Mater. 2005 Feb;21(2):129-38. doi: 10.1016/j.dental.2004.02.007. PMID: 15681011.
- Charig AJ, Thong S, Flores F, Gupta S, Major E, Winston AE. Mechanism of action of a desensitizing fluoride toothpaste delivering calcium and phosphate ingredients in the treatment of dental hypersensitivity. Part II: comparison with a professional treatment for tooth hypersensitivity. Compend Contin Educ Dent. 2009 Nov-Dec;30(9):622-4, 626, 628 passim. PMID: 19998729.
- Machado AC, Rabelo FEM, Maximiano V, Lopes RM, Aranha ACC, Scaramucci T. Effect of in-office desensitizers containing calcium and phosphate on dentin permeability and tubule occlusion. J Dent. 2019 Jul;86:53-59. doi: 10.1016/j.jdent.2019.05.025. Epub 2019 May 22. PMID: 31125585.
- Petersson LG. The role of fluoride in the preventive management of dentin hypersensitivity and root caries. Clin Oral Investig. 2013 Mar;17 Suppl 1(Suppl 1):S63-71. doi: 10.1007/s00784-012-0916-9. Epub 2012 Dec 28. PMID: 23271217; PMCID: PMC3586140.
- Gholami GA, Fekrazad R, Esmaiel-Nejad A, Kalhori KA. An evaluation of the occluding effects of Er;Cr:YSGG, Nd:YAG, CO2 and diode lasers on dentinal tubules: a scanning electron microscope in vitro study. Photomed Laser Surg. 2011 Feb;29(2):115-21. doi: 10.1089/pho.2009.2628. PMID: 21288081.
- Kim SY, Kim EJ, Kim DS, Lee IB. The evaluation of dentinal tubule occlusion by desensitizing agents: a real-time measurement of dentinal fluid flow rate and scanning electron microscopy. Oper Dent. 2013 Jul-Aug;38(4):419-28. doi: 10.2341/11-504-L. Epub 2012 Oct 30. PMID: 23110582.
- Marto CM, Baptista Paula A, Nunes T, Pimenta M, Abrantes AM, Pires AS, Laranjo M, Coelho A, Donato H, Botelho MF, Marques Ferreira M, Carrilho E. Evaluation of the efficacy of dentin hypersensitivity treatments-A systematic review and follow-up analysis. J Oral Rehabil. 2019 Oct;46(10):952-990. doi: 10.1111/joor.12842. Epub 2019 Jul 12. PMID: 31216069.
- Reis A, Dourado Loguercio A, Schroeder M, Luque-Martinez I, Masterson D, Cople Maia L. Does the adhesive strategy influence the post-operative sensitivity in adult patients with posterior resin composite restorations?: A systematic review and meta-analysis. Dent Mater. 2015 Sep;31(9):1052-67. doi: 10.1016/j.dental.2015.06.001. Epub 2015 Jun 27. PMID: 26122377.
- Castro AS, Maran BM, Gutiérrez MF, Martini EC, Dreweck FD, Mendez-Bauer L, Reis A, Loguercio AD. Dentin moisture does not influence postoperative sensitivity in posterior restorations: A double-blind randomized clinical trial. Am J Dent. 2020 Aug;33(4):206-212. PMID: 32794396.
- Arbildo-Vega HI, Lapinska B, Panda S, Lamas-Lara C, Khan AS, Lukomska-Szymanska M. Clinical Effectiveness of Bulk-Fill and Conventional Resin Composite Restorations: Systematic Review and Meta-Analysis. Polymers (Basel). 2020 Aug 10;12(8):1786. doi: 10.3390/polym12081786. PMID: 32785019; PMCID: PMC7464794.
- Schenkel AB, Peltz I, Veitz-Keenan A. Dental cavity liners for Class I and Class II resin-based composite restorations. Cochrane Database Syst Rev. 2016 Oct 25;10(10):CD010526. doi: 10.1002/14651858.CD010526.pub2. Update in: Cochrane Database Syst Rev. 2019 Mar 05;3:CD010526. PMID: 27780315; PMCID: PMC6461160.
- Chen C, Mao C, Sun J, Chen Y, Wang W, Pan H, Tang R, Gu X. Glutaraldehyde-induced remineralization improves the mechanical properties and biostability of dentin collagen. Mater Sci Eng C Mater Biol Appl. 2016 Oct 1;67:657-665. doi: 10.1016/j.msec.2016.05.076. Epub 2016 May 19. PMID: 27287165.
- de Oliveira ILM, Hanzen TA, de Paula AM, Perdigão J, Montes MAJR, Loguercio AD, Monteiro GQM. Postoperative sensitivity in posterior resin composite restorations with prior application of a glutaraldehyde-based desensitizing solution: A randomized clinical trial. J Dent. 2021 Dec 5;117:103918. doi: 10.1016/j.jdent.2021.103918. Epub ahead of print. PMID: 34879245.
- Sobral MA, Garone-Netto N, Luz MA, Santos AP. Prevention of postoperative tooth sensitivity: a preliminary clinical trial. J Oral Rehabil. 2005 Sep;32(9):661-8. doi: 10.1111/j.1365-2842.2005.01479.x. PMID: 16102079.
- Chermont AB, Carneiro KK, Lobato MF, Machado SM, Silva e Souza Junior MH. Clinical evaluation of postoperative sensitivity using self-etching adhesives containing glutaraldehyde. Braz Oral Res. 2010 Jul-Sep;24(3):349-54. doi: 10.1590/s1806-83242010000300015. PMID: 20877974.
- Sartori N, Lopes GC, Vieira LC. Clinical performance of cervical restorations with desensitizing agents: 18-month clinical trial. J Adhes Dent. 2012 Apr;14(2):183-9. doi: 10.3290/j.jad.a21542. PMID: 21734980.
About the Author
 Akram Sayed Ahmed is a resident in the Biomaterials program at the UAB School of Dentistry.
Akram Sayed Ahmed is a resident in the Biomaterials program at the UAB School of Dentistry.
 Krisha Shah is a resident in the Biomaterials program at the UAB School of Dentistry.
Krisha Shah is a resident in the Biomaterials program at the UAB School of Dentistry.
 Dr. Nate Lawson is an Associate Professor and Director of the Division of Biomaterials at the UAB School of Dentistry. Additionally, he is the director of a 2-year Biomaterials residency program. He is passionate about testing new materials and techniques in the laboratory and applying the results to clinical dentistry. His primary research interests are related to the physical and mechanical properties of dental ceramics, composites, cement, and adhesives.
Dr. Nate Lawson is an Associate Professor and Director of the Division of Biomaterials at the UAB School of Dentistry. Additionally, he is the director of a 2-year Biomaterials residency program. He is passionate about testing new materials and techniques in the laboratory and applying the results to clinical dentistry. His primary research interests are related to the physical and mechanical properties of dental ceramics, composites, cement, and adhesives.












