Abstract
Tooth autotransplantation is defined as the surgical movement of a tooth from one alveolar socket to another in the same individual. It is a viable option to replace teeth lost or having a poor prognosis.
The treatment plan included extracting tooth No. 2, which was fractured, and transplanting No. 1 after using a 3-D replica, instead of using the donor tooth as the guide to prepare the recipient alveolar socket.
Radiographic and clinical controls showed complete periradicular bone healing, and a tomographic control at 3 years showed 3-D healing of the attachment apparatus including a new buccal cortical bone plate and lamina dura on the sinus floor.
Tooth autotransplantation is a predictable procedure with high success and survival rates; therefore, this procedure should be taken into account as an alternative to replace lost or nonrestorable teeth or those having a poor prognosis.
Introduction
Tooth autotransplantation, defined as the surgical movement of a tooth from one alveolar socket to another, either after extraction or by surgically preparing the recipient site in the same patient, is a viable option to replace teeth lost or having a poor prognosis. Thus, both function and preservation of the alveolar bone crest is maintained. Its success is associated with healing of the periodontal ligament (PDL) and pulp tissue, absence of root resorption, soft tissue healing, and radicular formation.1-3
Autotransplantation was first documented by French dentist Pierre Fauchard in his book, Le Chirurgien Dentiste, in 1798.4,5 In the early 1950s, the procedure became popular to replace badly decayed first molars by impacted third molars with incomplete root formation, but the idea lost popularity due to its low success rate (around 50%).6 However, clinical and experimental studies in the past 40 years have shown that autotransplantation can be a predictable treatment option.5,7,8
Indications include ectopic or impacted teeth; premature tooth loss due to trauma, tumors, iatrogenic reasons, dental agenesis; or to replace teeth with poor prognosis or developmental abnormalities.9
Dental autotransplantation also has some clear advantages, such as allowing tooth relocation to distant sites in the same or opposite arch as well as from the mandible to the maxilla and vice versa. It induces bone formation and re-establishes a normal bone socket.10
Third molar autotransplantation with either complete or incomplete root formation used to replace nonrestorable or absent first or second molars has proven to be a convenient and predictable procedure, especially during growth, with a success rate above 86%.11
Most studies have focused on autotransplanting teeth with incomplete root formation,12 which limits the technique to either adolescents or young adults.10
In recent years, teeth with complete root formation have also been transplanted, expanding the potential role of the technique. Recent meta-analyses have demonstrated that there is no significant difference in success or survival rates between autotransplanted teeth with complete or incomplete root formation. In 2014, Chung et al. reported a success rate of 98% at 1 year and 90.5% at 5 years in teeth having complete root formation, with resorption occurring in 2% or less of the cases.13 Rohof et al. in 2018 reported a survival rate at 1 and 5 years higher than 97%, and at 10 years, 96.3%.14
In conventional autotransplantation techniques, the donor tooth serves as the guide to prepare the recipient alveolar socket. This means that the donor tooth is usually manipulated and handled through many attempts in order to obtain an optimal adaptation between the alveolar bone and the radicular surface of the transplanted tooth. This technique results in a higher risk of trauma to the PDL as well as increasing the extra-alveolar time. In modern techniques, instead of using the donor tooth for that purpose, damage to the donor tooth is minimized by the use of a preoperatively designed surgical guide. This replica can be manufactured based on a cone-beam computed tomography (CBCT) scan of the donor tooth, in which the digital imaging and communications in medicine (DICOM) files can be used to segment the images of the tooth away from those of the bone, nearby teeth, and soft tissues. Then the images are saved as stereo lithography (STL) files, so that an STL impression can be manufactured in a high definition 3-D printer. During the surgical procedure, the 3-D printed replica serves as a guide by using it in the recipient alveolar socket, facilitating an autotransplantation procedure that is faster and easier.15
Case Report
A 52-year-old male patient was referred for evaluating his maxillary right second molar (tooth No. 2), reporting having had pain upon biting on the area for the past 6 months. A diagnosis of vertical root fracture had already been confirmed by another endodontist during exploratory surgery. He had offered the patient tooth extraction and replacement by a fixed bridge using the first and third molars as abutments. Other options were extraction and implant placement. However, the patient opted for another opinion because he was discouraged by the delay in the final restoration (1 year), as offered.
Clinical examination revealed a large composite restoration; the patient reported pain to the vertical percussion. The tooth had normal mobility and 10-mm pockets on both the palatal and buccal surfaces.
Panoramic and periapical radiographs showed a molar with fused roots, a large single canal, and a periapical radiolucency, Small field-of-view CBCT (Planmeca ProMax 3-D Classic; Planmeca, Helsinki, Finland) revealed complete bone loss of the buccal cortical plate, cervical bone loss of the palatal cortex, cortical bone loss of the maxillary sinus, mucosal thickening of the sinus membrane (mucositis), and hypodense areas on the sagittal and axial views. These radiographic, tomographic, and clinical findings were consistent with the previous diagnosis of vertical root fracture. (Fig. 1)
Fig. 1
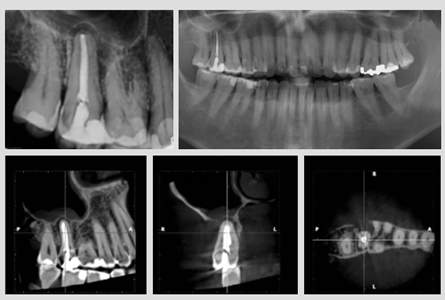
Clinically and radiographically, the maxillary right third molar (No. 1) was fully erupted and in its correct position in the dental arch. It seemed to have only one root and a very small class I composite resin restoration, so it was considered a good candidate for autotransplantation. The treatment plan included extracting No. 2 and transplanting No. 1 after using a 3-D replica of No. 1 so as to reduce extra-alveolar time and make the necessary adjustments in the recipient alveolar socket before transplanting the donor tooth. The patient agreed to the treatment plan and signed an informed consent.
Using the DICOM format, the CBCT volumes were exported into the software used for medical image informatics, image processing, and three-dimensional visualization (3-D Slicer, Brigham Health, Harvard Medical School, Boston, Mass, USA). Root length and cervical dimensions of the donor tooth were measured and compared with the residual alveolar bone crest height, width of the recipient site, and anatomical relation with the maxillary sinus to segment the images of the donor tooth once separated from the bone, other teeth, and adjacent soft tissues. The images were then saved as STL files, allowing for a stereolithographic reproduction in a 3-D, high definition printer (Objet30, Stratasys Ltd, Eden Prairie, MN, USA), with a biocompatible resin (Verodent Plus, Stratasys). During the surgical procedure, this autoclaved replica functioned as a guide when inserting it in the recipient site, allowing a more efficient procedure. (Fig. 2)
Fig. 2

The surgical procedure consisted of disinfection and anaesthesia with 2% lidocaine with 1:80000 epinephrine (New Stetic, Guarne, Ant. Colombia) of both surgical sites, atraumatic extraction of No. 2 by passive use of forceps, (Figs. 3A,B) and confirming the adaptation of the 3-D replica in the recipient site. Because the transplanted tooth should have 1-2 mm of free play in the alveolar socket, (Fig. 3C), some modifications with surgical burs (All Port Bone E-cutter carbide; Brasseler, Savannah, Ga, USA) were needed. Then No. 1 was also atraumatically extracted with forceps, minimizing the use of surgical elevators to prevent damage to the PDL and radicular cementum.
The donor tooth was then positioned in the recipient site and splinted with a semirigid orthodontic wire for 4 weeks. Total extra-alveolar time was 5 s. (Fig. 3D)
Fig. 3A-B

Fig. 3C

Fig. 3D

Fig. 3E

Occlusal adjustments were done to keep the tooth in hypo-occlusion. Pre-, during, and posttransplantation radiographs were taken to evaluate the position of the transplanted tooth in the alveolar socket. Azithromycin (500 mg tablets) q 24 h and Nimesulide tablets (100 mg) q 12 h were prescribed for 3 days. Careful oral and written instructions were given to the patient for postoperative care, which included chlorhexidine rinses for 1 week.
Endodontic treatment of the transplanted tooth was performed 2 weeks after the surgical procedure to avoid weakening the dental structure before the transplantation and to allow PDL and soft tissue healing. Based on the CBCT examination, which determined that the tooth had only one root with one root canal, a conservative access cavity was done and root canal therapy was performed with Protaper Next instruments (Dentsply Sirona, Ballaigues, Switzerland). Then irrigation was done with 5 mL NaOCl 5.25% between each instrumentation and final rinses with 5 mL 17% EDTA and distilled water. The cavity was filled with a warm vertical compaction of gutta-percha and Topseal (Dentsply Sirona), then the small class I access cavity was sealed with composite resin.
The splint was removed after 4 weeks to assess stabilization and mobility of the tooth, then clinical and radiographic follow-ups were done at 1, 3, 6, 12, and 36 months. A tomographic follow-up was done at 36 months, where good periodontal healing was observed, with mobility being similar to that of the first molar. Periodontal probing was done after 3 months and in all the subsequent recalls, where no bleeding and normal probing depths of 3 mm around the tooth remained throughout the recall appointments.
Radiographically, complete periradicular bone healing was observed, and a tomographic control at 3 years showed 3-D healing of the attachment apparatus including a new buccal cortical bone plate and a lamina dura on the sinus floor. No further sinus mucositis was observed. (Fig. 4).
Fig. 4

D. Axial plane. Note a new buccal cortical plate, maxillary sinus floor plate, and absence of mucositis.
Discussion
In recent years, tooth autotransplantation has begun to resurge as a promising treatment option, mainly because of the rise in research on PDL healing.
The most critical factor for success in autotransplantation is the presence of a viable PDL on the root surface; with no difference of whether the tooth presents complete or incomplete root formation.16
If the PDL is compromised or damaged during the surgical procedure or during the healing phase, many types of resorption can occur, including superficial resorption (SR), inflammatory resorption (IR) replacement resorption (RR), or invasive cervical resorption (ICR).5
SR is self-limiting and does not require treatment. IR is caused by infection of the root canal system and damage to the PDL. Because IR occurs in the first 3 months after transplantation and progresses quickly independent of the patient’s age, a radiograph must be taken each month during the first 3 months to monitor possible signs of IR. If IR is detected, root canal treatment should be initiated as soon as possible; the IR will heal if the resorption area is small.17,18
For autotransplanted teeth with completely formed roots, endodontic treatment is usually indicated 2 weeks after the intervention,10 which is the way the present case was handled.
Replacement resorption cannot be treated because it is a healing phase; eventually, the roots will be resorbed and replaced by bone. However, in adult patients, this happens slowly and the teeth can remain functional for a long time.
The most prevalent cause of failure of autotransplanted teeth is RR, which happens more often in older patients;5 however, in this case, there was no clinical, radiographic, or tomographic evidence of it.
ICR is a dynamic process that arises in the cervical area of the tooth, and because of the action of odontoclasts, ICR results in the loss of radicular tooth structure. Its mechanism is not fully understood, and the treatment can be surgical exposure and sealing of the defect when it is supracrestal and superficial. In more extensive lesions, this procedure can be complemented with root canal treatment. In some instances, the only way to seal nonapproachable defects is via surgery; intentional replantation is also an alternative.19
One advantage of autotransplantation is bone healing. The viable PDL of the transplanted tooth can induce and maintain alveolar bone. Stem cells residing in the PDL can differentiate into three types of cells: fibroblasts, cementoblasts, and osteoblasts. Differentiated osteoblasts can form bone around the transplanted tooth20 and in such cases, bone apposition and regeneration can be observed in the recipient alveolar socket along with lamina dura around the transplant. Maxillary bone growth is related to tooth eruption, and bone morphology is partially maintained by teeth.
The PDL plays an important role in bone remodelling, and it is also critically important in the changes and movements of the dentition throughout life. Many biological aspects of bone formation are regulated by the PDL, so this formation is expected to happen in a normal way in transplanted teeth.5
Many benefits of using 3-D replicas of the donor tooth have been reported; these replicas allow for the receptor site to be outlined, thus they avoid unnecessary damage to the PDL because the donor tooth is not being pushed into the alveolar socket to be adapted into it. It efficiently reduces extra-alveolar time, which improves viability of the PDL cells, because it has been shown that PDL-cell viability is dramatically reduced after 18 min of extra-alveolar time.15
In 2019, EzEldeen et al. did a prospective clinical trial wherein 100 autotransplanted teeth in 88 patients were followed up for 10 years. Equal numbers of teeth were divided into an experimental group, in which the procedure was guided by using CBCT and a 3-D replica, and a control group in which radiographs and conventional techniques were used. In the results, even though there were no statistically significant differences, there was a higher tendency for success and survival to occur in the experimental 3-D guided group (92% and 86%, respectively) compared with a control group in which conventional techniques were used (84% and 78%, respectively).21
Zufía et al22 reported on a case similar to the present one wherein a mandibular second molar with considerable bone loss was replaced by a third molar that was transplanted with the buccal cortical bone adhering to it so as to confer on it a better initial stability and to regenerate the buccal cortex. The case was followed for 2 years, during which good clinical and radiographic results were obtained, but no CBCT was used.
In the present case, no bone regeneration procedures were used, but healthy radicular cement and PDL were sufficiently capable of regenerating a viable cortical plate, both in height and width, along with a normal sinus floor, which was evidenced in the CBCT images at the 3-year follow-up.
Oral Health welcomes this original article.
References
- Tsukiboshi M. Autotransplantation of teeth: requirements for predictable success. Dent Traumatol 2002; 18:157-80.
- Park JH, Tai K, Hayashi D. Tooth autotransplantation as a treatment option: A review. J Clin Pediatr Dent 2011;35: 129–136.
- Intra JB, Roldi A, Brandão RC, de Araújo Estrela CR, Estrela C. Autogenous premolar transplantation into artificial socket in maxillary lateral incisor site. J Endod 2014;40:1885-1890.
- Pape HD, Heiss R. History of tooth transplantation. Fortschr Kiefer Gesichtschir 1976;20:121–5.
- Tsukiboshi M. Long-term outcomes of autotransplantation of teeth: A case series. Dent Traumatol 2019;00:1–10.
- Natiella JR, Armitage JE, Greene GW. The replantation and transplantation of teeth. A review. Oral Surg Oral Med Oral Pathol 1970;29:397-419.
- Andreasen JO, Paulsen HU, Yu Z, Bayer T, Schwartz O. A long term study of 370 autotransplanted premolars. Part II. Tooth survival and pulp healing subsequent to transplantation. Eur J Orthod 1990;12:14–24.
- Andreasen JO, Paulsen HU, Yu Z, Bayer T. A long term study of 370 autotransplanted premolars. Part IV. Root development subsequent to transplantation. Eur J Orthod 1990;12:38–50.
- Almpani K, Papagiorgiou S, Papadopoulos M. Autotransplantation of teeth in humans: a systematic review and meta-analysis. Clin Oral Investig 2015;19:1157-79.
- Abella F, Ribas F, Roig M, Gonzalez J, Duran-Sindreu F. Outcome of Autotransplantation of Mature Third Molars Using 3-dimensional–printed Guiding Templates and Donor Tooth Replicas. J Endod 2018;44:1567-74.
- Nagori SA, Bhutia O, Roychoudhury A, Pandey RM. Immediate autotransplantation of third molars: an experience of 57 cases. Oral Surg Oral Med Oral Pathol Oral Radiol 2014;118:400-7.
- Bauss O, Zonios I, Rahman A. Root development of immature third molars trans- planted to surgically created sockets. J Oral Maxillofac Surg 2008;66:1200-11.
- Chung W-C, Tu Y-K, Lin Y-H, Lu H-K. Outcomes of autotransplanted teeth with complete root formation: a systematic review and meta-analysis. J Clin Periodontol 2014; 41: 412–423.
- Rohof ECM, Kerdijk W, Jansma J, Livas C, Ren Y. Autotransplantation of teeth with incomplete root formation: a systematic review and meta-analysis. Clin Oral Investig 2018;22:1613-24.
- Verweij JP, Jongkees FA, Anssari Moin D, Wismeijer D, van Merkesteyn JPR. Autotransplantation of teeth using computer-aided rapid prototyping of a three-dimensional replica of the donor tooth: a systematic literature review. Int J Oral Maxillofac Surg 2017;46:1466-74.
- Andreasen JO. Relationship between cell damage in the periodontal ligament after replantation and subsequent development of root resorption. Acta Odontol Scand 1981;39:15–25.
- Andreasen JO. Relationship between surface and inflammatory resorption and changes in the pulp after replantation of permanent incisors in monkeys. J Endod 1981;7:294–301.
- Andreasen JO. The effect of pulp extirpation or root canal treatment on periodontal healing after replantation of permanent incisors in monkeys. J Endod 1981;7:245–52.
- Patel S, Foschi F, Condon R, Pimentel T, Bhuva B. External cervical resorption: part 2–management. Int Endod J 2018;51:1224-38.
- Inoue T, Chen SH, Usuda J, Morohoshi Y, Shimono M. Osteogenic activity of cells from dental pulp, periodontal ligament, bone marrow and muscle in vitro: an ultrastructural study and alkaline-phosphatase activity. Bull Tokyo Dent Coll 1992;33:7-12.
- EzEldeen M, Wyatt J, Al-Rimawi A, Coucke W, Shaheen E, Lambrichts I, Willems G, Politis C, Jacobs R. Use of CBCT Guidance for Tooth Autotransplantation
in Children. J Dent Res 2019;98:406-13. - Zufía J, Abella F, Trebol I, Gómez-Meda R. Autotransplantation of Mandibular Third Molar with Buccal Cortical Plate to Replace Vertically Fractured Mandibular Second Molar: A Novel Technique. J Endod 2017;43:1574-78.
About the Authors
 Dr. Felipe Restrepo, born in Medellín, Colombia, from the Universidad de Antioquia with a degree in Dentistry. Graduate of a two-year Endodontic program at Universidad CES. Associate Professor at the Universidad de Antioquia and Director of the Dental Emergencies Diploma. Former president of the Antioquian Association of Endodontists. Private practice limited to endodontics and endodontic microsurgery in Medellín. felipe.restrepo@udea.edu.co.
Dr. Felipe Restrepo, born in Medellín, Colombia, from the Universidad de Antioquia with a degree in Dentistry. Graduate of a two-year Endodontic program at Universidad CES. Associate Professor at the Universidad de Antioquia and Director of the Dental Emergencies Diploma. Former president of the Antioquian Association of Endodontists. Private practice limited to endodontics and endodontic microsurgery in Medellín. felipe.restrepo@udea.edu.co.
 Dr. Paula Villa is an endodontist from Universidad CES in Medellin. She is cofounder of the graduated endodontic program in Universidad de Antioquia, where she is Associate Professor. Former president of the Colombian Association of Endodontists. Private practice is limited to endodontics and endodontic microsurgery in Medellín, Colombia.
Dr. Paula Villa is an endodontist from Universidad CES in Medellin. She is cofounder of the graduated endodontic program in Universidad de Antioquia, where she is Associate Professor. Former president of the Colombian Association of Endodontists. Private practice is limited to endodontics and endodontic microsurgery in Medellín, Colombia.
 Dr. David Arango is a periodontist from Pontificia Universidad Javeriana. Professor of periodontics at Universidad de Antioquia and Director of the Osseointegration Diploma. Private practice is limited to periodontics, periodontal plastic purgery, bone regeneration and dental implants. david.arango@udea.edu.co.
Dr. David Arango is a periodontist from Pontificia Universidad Javeriana. Professor of periodontics at Universidad de Antioquia and Director of the Osseointegration Diploma. Private practice is limited to periodontics, periodontal plastic purgery, bone regeneration and dental implants. david.arango@udea.edu.co.
 Dr. Jorge Vera Graduated from the National University of Mexico, Postgraduate Endodontic Certificate from Tufts University, School of Dental Medicine, Boston, MEndod from Yury Kuttler institute Mexico. Certificate of achievement on fundamentals-Pharmacology from Harvard Medical School. Professor of Endodontics University of Tlaxcala, Mexico. Private practice limited to endodontics.
Dr. Jorge Vera Graduated from the National University of Mexico, Postgraduate Endodontic Certificate from Tufts University, School of Dental Medicine, Boston, MEndod from Yury Kuttler institute Mexico. Certificate of achievement on fundamentals-Pharmacology from Harvard Medical School. Professor of Endodontics University of Tlaxcala, Mexico. Private practice limited to endodontics.












