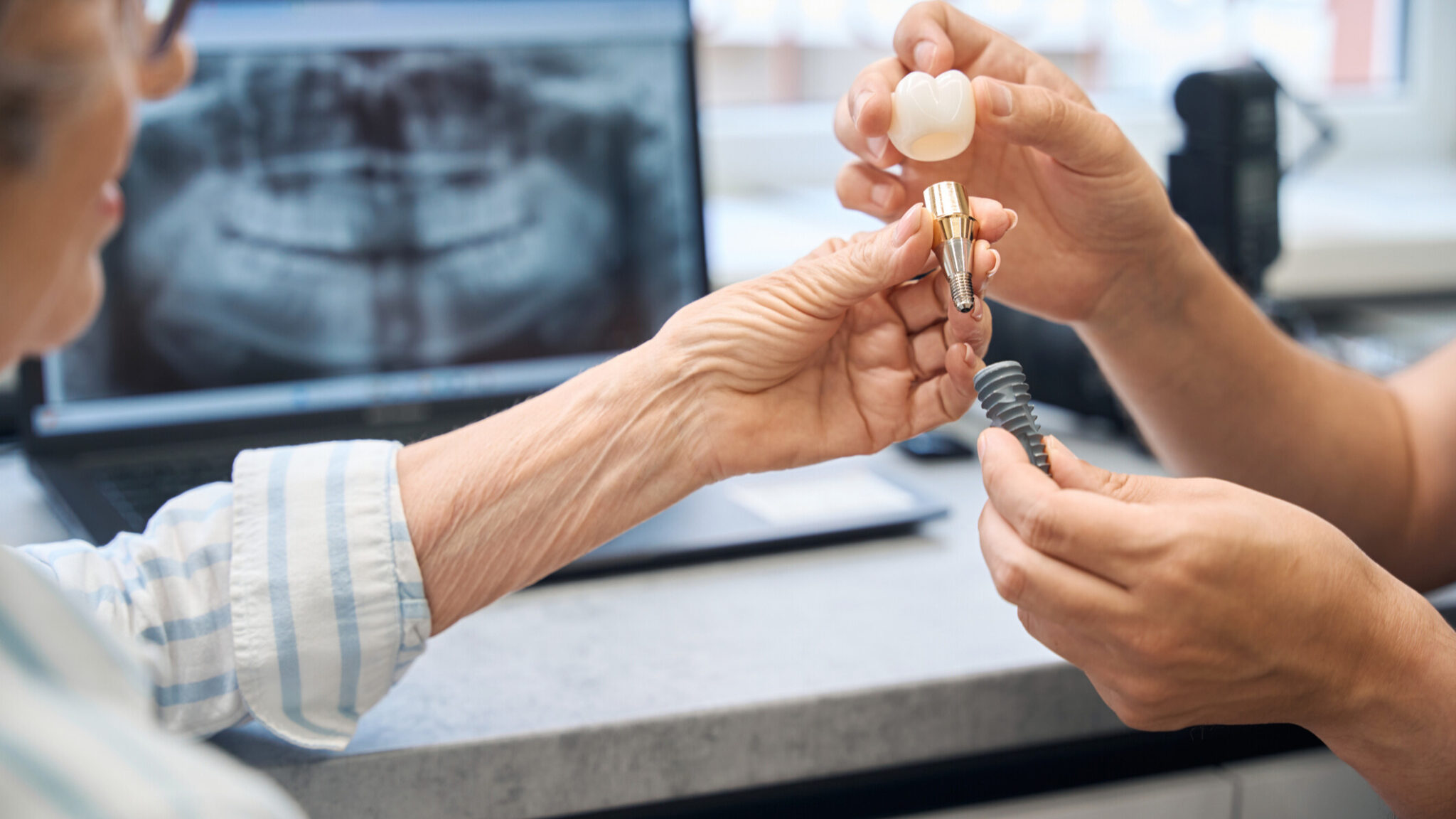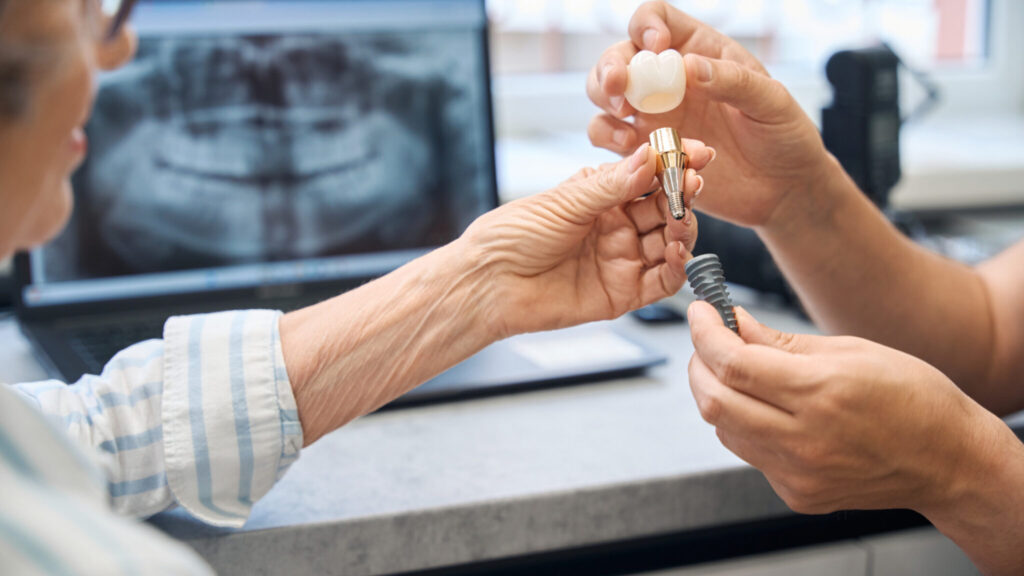
The U.S. Food and Drug Administration has finalized guidance for manufacturers of dental bone grafting material devices, with the goal of reducing reliance on animal studies.
The document outlines recommendations to help manufacturers comply with special controls for these devices and increase transparency around animal testing.
“The guidance also provides recommendations that may aid in the reduction of the total number of animals used to support a submission, supports the 3Rs of replace, reduce and/or refine animal use,” the FDA said.
It also encourages manufacturers to consult with the agency if they wish to use a non-animal testing method they believe is suitable.
Related: Plasma rich in growth factors for bone grafting: A paradigm shift
Bone grafting on the increase
Bone grafting has become increasingly common due to advances in regenerative medicine, new biomaterials and growing patient demand for restoring lost bone.
A 2021 research paper estimated that up to 50 per cent of all dental implant procedures involve the use of bone grafts. Globally, about 2.2 million bone graft procedures are performed each year, with an estimated cost of US$664 million in 2021. The number of operations to repair bone defects is projected to grow roughly 13 per cent annually. Meanwhile, the gold standard remains autografting, which uses a patient’s own bone for dental implantation, reconstruction and repair of the mouth, face and skull.
Animal-derived materials, known as xenografts, are widely used in dental bone grafting procedures.
Bovine and porcine bones — from cows and pigs — are the most common sources, according to research published in PeerJ and ScienceDirect.
Other animals, including horses, camels and even ostriches, have also been used, though less frequently. Studies and reviews have documented their use in specific cases.
Bone grafting has a long history. The first successful bone transplant was reported in 1668, when Dutch surgeon Job van Meekeren transplanted a piece of canine skull into the head of a Russian soldier, achieving full integration.