
Dental implants to replace missing teeth are a predictable treatment option with survival rates ranging as high as 98 to 99%.1-6 Antiresorptive agent-related osteonecrosis of the jaw (ARONJ) is a serious dental surgical post-operative risk for patients on antiresorptive medications, and may also interfere with dental implant osteointegration. The data on antiresorptive medication-associated implant failure and implant failure associated with ARONJ have been conflicting and limited.
Antiresorptive medications
Antiresorptive medications were prescribed for malignancies like metastatic bone tumors, cancers, and multiple myeloma; for bone diseases like osteoporosis, osteopenia, Paget’s disease; and for medication-associated bone resorption from antineoplastic agents, thyroid supplements, Lupron therapy for prostate cancer, glucocorticoids, and antacids.7 Antiresorptive medications including bisphosphonates (BPs) and denosumab increase bone density by decreasing osteoclastic activity.
On a cellular level, BPs reduce osteoclastic activity while osteoblasts continue bone deposition, resulting in net cortical bone gain. BPs disrupt the normal osteoclastic action, causing retraction of the normal ruffled border from the bone surface, the ensuing osteoclast death results in no new osteoid formation.8 In addition, BPs can also inhibit angiogenesis.9-13 The reduced angiogenesis may have an adverse effect on the dental implant osseointegration. Furthermore, the BP induced avascular and acellular bone may be less able to resist microbial invasion, increasing the risk for peri-implant bone loss or osteonecrosis.
Oral BPs have been used for treating osteoporosis and osteopenia.14 Intravenous (IV) BPs have been used for treating hypercalcemia-associated bone diseases.15-17 The effect of BPs on the osteoclasts depends on the chemical structure, non-nitrogen, or nitrogen-containing BP. Nonnitrogen BP include tiludronic acid, etidronate disodium, and clodronate disodium. Osteoclasts metabolize non-nitrogen BP producing cytotoxic analogs of adenosine triphosphosphates, causing apoptosis of osteoclast and impairing resorption. As for osteoclasts metabolism of nitrogen-containing BP, the mevalonate pathway disruption interferes with bone resorption by inhibiting posttranslational protein and osteoclast’s ruffled border formation.18,19 Nitrogen-containing BPs show stronger antiresorptive activities than non-nitrogen containing BPs.20 BPs have high affinity for hydroxyapatite and accumulate in the bone matrix for an extended period.
Denosumab, a human monoclonal antibody, has been used for treating osteoporosis and bone metastasis.21,22 Denosumab increases bone density by inhibiting osteoclastic activity and reducing bone resorption.21 This highly specific action inhibits receptor activator of nuclear factor-kappa B ligand (RANKL).23 RANKL regulates osteoclasts survival, differentiation and function. Denosumab, a RANKL neutralizing antibody, has been shown to reduce skeletalrelated pathological fractures, spinal cord compression, or hypercalcemia in cases of metastatic bone cancer.24 Denosumab also suppresses osteoclasts differentiation from osteoclast precursors expressing RANK.24
BPs are most commonly reported in conjunction with dental implant placement (Table 1). The most common indication for BPs use in these studies was osteoporosis. More females than males were taking BPs for osteoporosis. This is because BPs have been effective in increasing bone mineral density and reducing fractures.7
Table 1: Antiresorptive Medications and Implant Success (Clinical studies)
| Study | No. of BP patients (Male/ Females) | Indications (Medication: no. of patients) | Duration of medication before implants (no. of patients) | ARONJ due to implant surgery | Success rate of implants in medication group | Success rate of implants incontrol group | Manage- ment of implant failure | Bone grafting | Antibiotic therapy | Mouth rinse |
| Al-Sabbagh et al. 201546 | 20 (1/19) 46 implants | Osteoporosis (Oral BP) | > 3 yrs | None | Implant level: 100% Patient level: 100% | Implant level: 100% Patient level: 100% | No implant failure | NR | NR | NR |
| Bell and Bell 200842 | 42 (2/40) 100 implants | Osteoporosis (Alendronate: 34, Risedronate: 6, Ibandronate: 2) | 6 mos – 11 yrs | None | Implant level: 95% | Implant level: 96.5% | All 5 implant failure had subsequent implant replaced successfully | 30 patients Grafting materials: autogenous bone from intraoral sites, xenograft 68 bone graft procedures: 41 socket graft, 6 sinus lifts, 4 closed lifts, 14 GTR, 1 tunnel graft, 3 buccal contour graft 1 case of bone graft failure | NR | NR |
| Famili et al. 201145 | 22 (0/22) 75 implants | Osteoporosis (Oral BP: Fosamax: 15, Boniva: 1, Fosamax to Boniva:1, Boniva to fosamax:1, Actonel: 4) | 6 mos – 1 yr : (6) > 1 yr : (9) > 5 yrs : (5) NR: (1) | None | Implant level: 98.7% | No control | Successful replacement of implant 1 year later | NR | NR | NR |
| Fugazzotto et al. 200740 | 61 (0/61) 169 implants | Indication not reported (Oral BP Aldendronate 35 mg: 33, Aldendronate 70 mg: 18, Risedronate 35 mg: 7, Risedronate 70 mg: 3) | 1-5 yrs Mean: 3.3 yrs | None | Implant level: 100% Patient level: 100% | NR | No implant failure | Particulate bone graft: 1 patient had exposed bone on torus after immediate implant, which resolved after debridement | Amoxicillin 500 mg TID for 10 days or erythromycin 333mg TID for 10 days if allergic Etodolac 400 mg TID for 5 days, as needed | Post-op CHX rinse BID for 21 days |
| Grant et al. 200844 | 89(0/89) 468 implants | Indication not reported (Oral BP) | Mean: 38 mos >3 yrs: (33) <3 yrs: (56) | None | Implant level: 99.6% | None | 2 implant failure, 1 redone successfully, 1 was not replaced | 1 of the 2 Failed implants case had left maxillary sinus lift 32 of 115 patients had sinus augmentation | NR | NR |
| Jeffcoat 200638 | 25 (0/25) 102 implants | Indication not reported (Oral BP: Alendronate Risendronate) | 1-4 yrs (mean: 3 yrs) | None | Implant level: 100% Patient level: 100% Compared to control: no statistical significance | Implant level: 99.1% Patient level: 96% | No implant failure | NR | NR | NR |
| Kasai et al. 200941 | 11 (0/11) 35 implants | Osteoporosis (Oral BP: Alendronate, Fosamax ) | None | None | Implant level: 85.7% | Implant level: 95.7% Patient level: NR | Failed implants removed, no report of replacement | NR | NR | |
| Koka et al. 201035 | 55 (0/55) 121 implants | Osteoporosis or osteopenia (BP NR) | <3 yrs: (16) 3-5 yr: (20) >5 yr: (19) | None | Implant level: 99.2% Patient level: 98.2% | Implant level: 98.2% Patient level: 97.6% | NR | NR | NR | NR |
| Memon et al. 201243 | 100 (0/100) 153 implants | Indication not reported (Oral BP: Alendronate: 72, Risdronate: 23, Ibandronate: 5, IV BP: excluded) | < 1 yr : (2) 1-3 yrs : (19) > 3 yr : (15) NR: (46) | None | Implant level: 93.5% | Implant level: 95.5% | NR | 44 patients had bone grafting | 44 patients had bone grafting | NR |
| Mozzati et al. 201536 | 235 (0/235) 1267 implants | Osteoporosis (Oral BP) Exclusion: Current or past history of IV BP | NR for all subjects | None | Implant level: 98.7% Patient level: 93.2 % | NR | Failed implants were successfully replaced | 54 patients needed sinus lifts Strong association of sinus lift and implant failures | 54 patients needed sinus lifts Strong association of sinus lift and implant failures | NR |
| Siebert et al. 201526 | 12 (0/12) 60 implants | Osteoporosis (IV Zoledronic acid, annual infusions 5 mg) | 2-3 yrs | None | Implant level: 100% Patient level: 100% No statistical difference between experimental group and control | Implant level: 100% Patient level: 100% | No implant failure | 1g amoxicillin/ clavulanic acid BID for 6 days, starting 24 hours prior to surgery | 1g amoxicillin/ clavulanic acid BID for 6 days, starting 24 hours prior to surgery | |
| Tallarico et al. 201539 | 32 (0/32) 98 implants | Indication not reported (Oral BP: Aldendronate 70 mg) | > 3 yrs | None | Implant level: 98.98% | NR | 3 patients had peri- implant mucosal inflammation after 6 mos Improved oral hygiene reduced peri-implant inflammation | NR | NR | CHX 0.2%: 7 day before surgery, day of surgery, and 7 days after surgery |
| Yajima et al. 201748 | 11 (0/ 11) 25 implants | Osteoporosis (BP not specified) | 1-3 yrs: (5) > 3 yrs: (6) | None | Implant level: 88.9% Patient level: 72.7% | Implant level: 100% Patient level: 100% | NR | NR | NR | NR |
| Zahid et al. 201118 | 26 (1/25) 51 implants | Osteoporosis (BP not specified) | Incomplete data set | None | Implant level: 94.1% Patient level: 88.5% | Implant level: 97.1% | 3 failed implants, 2 implants replaced successfully, 1 was not replaced | NR | NR | 0.5 oz of 0.12% CHX 4 times a day for 2 wks post- |
ARONJ
Nitrogen-containing BPs have commonly been associated with implant failure and ARONJ.18 IV administration of BPs has also been associated with an increased risk of ARONJ.25 ARONJ (Fig.1 & Fig.2) associated with BPs manifest as painful exposed bone, necrotic bone, and intraoral or extraoral fistulas that do not heal within 8 weeks.26,27 Patients taking BPs needing dental extractions have reported higher risks of ARONJ.28-33 The ARONJ risk of patients taking BPs needing dental implants has been unpredictable.29,33,34 Thus, BP use may be a contraindication to elective surgical procedures, including dental implant placement.29,35 Due to limited data, the American Association of Oral and Maxillofacial Surgeons stated that the risk of ARONJ after dental implant placement may be similar to the risk after dental extractions.27 However, there are conflicting reports of dental implant success without the occurrence of ARONJ.35-37
Fig. 1
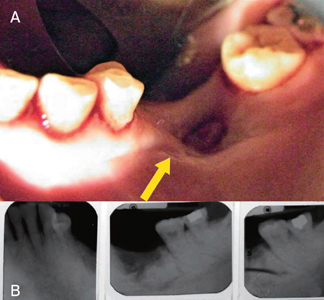
Fig. 2

Dental implant survival in patients on BPs
Table 1 included clinical studies on patients on BPs requiring dental implant surgery, 3 were prospective studies 26,38,39 and the other 11 were retrospective studies. The use of antibiotics pre-operative, intra-operative, or post-operative may influence ARONJ and dental implant infections. Six studies18,26,36,39-41 reported prophylactic antibiotics before and 5-10 days after dental implant surgery, the remaining studies did not report antibiotic use. Antibiotics reporting could have been overlooked if antibiotics were used routinely. Only 1 study40 reported the use of chlorohexidine mouthwash postimplant surgery, the rest of the studies did not report the use of postoperative mouthwash.
Bone grafting for site development before dental implant surgery was performed in the 5 studies,36,40,42-44 one study42 reported bone grafting failure, 2 studies reported implant failure associated with sinus lifts,36,44 and remaining 2 studies did not report on the data.40,43 Since osteoclasts are integral for graft turnover during healing, BP-related osteoclast apoptosis may decrease bone grafting success.13 However, this was not apparent as only 2 out of the 5 studies reported grafting failure. The two studies36,44 with bone graft failure reported that the graft failure did not progress to osteonecrosis. These implant site development procedures performed included sinus lifts, guided bone regeneration, ridge preservation, and ridge augmentation. The bone graft materials used included combinations of autografts and allografts together or alone.
Six studies36,39,40,42,44,45 reported complications like periimplant inflammation, mobility, implant failure, or exposed bone. However, no studies reported ARONJ as an outcome of dental implant surgery. Only one study38 reported no adverse reactions. Implant survival was 85.7-100%. Four studies26,38,40,46 did not have any implant failures in the BP group. However, patients taking BPs may have a greater risk of peri-implantitis and peri-implant bone loss.18 Dental implant-related wound healing requires alveolar metabolic and physiologic changes around the implant for successful integration of the dental implant to the alveolar bone. Antiresorptive medication alters the bone physiology (Fig.3). It decreases osteoclastogenesis, increases osteoclasts apoptosis, decreases bone resorption, decreases osteoblastogenesis, and decreases bone formation.19
Fig. 3

Implant failure has been reported as osteointegration failure.47 BPs may affect initial implant integration at the osteoconduction phase which forms new bone at the boneimplant interphase.47 BPs can persist for years in the bone matrix, and can affect every aspect of bone remodeling. BPs may be released during bone remodeling, and embedded into new bone.7 BP-related bone turnover suppression is associated with cumulative of bone microdamage and excessive occlusal forces, leading to increased bone resorption.13
Risks of implant failure in patients on BPs
Eight18,26,35,38,41-43,48 studies in Table 1 reported implantlevel data, only one study reported statistically significant difference in implant failure between the antiresorptive medication group and the control.41 In the antiresorptive medication group, 4.2% (27 of 647) of implants failed compared to 3.0% of implants (62 of 2056) in the control group.
Five26,35,38,46,48 studies in Table 1 reported patient-level data, none of the studies reported any statistically significant difference in implant failure between the antiresorptive medication group and control. In the antiresorptive medication group, 3.3% (4 of 123) of patients reported failed implants compared to 2.1% (3 of 142) of patients in the control group.
Other risk factors or medical comorbidities can increase implant failure and ARONJ risks. Besides taking BPs, smoking and radiotherapy were already significantly associated with implant failure. Other health conditions like osteoporosis and diabetes may have some reported effects on implant failure, which may not be statistically significant.49 Other factors for implant failure may be related to the alveolar position of the implant, there is increased implant failure in the maxilla50 especially the posterior maxilla.42
Osteoporosis and being female may also be co-factors contributing to the antiresorptive medication-associated risk for implant failure. There is a large majority of females with osteoporosis taking antiresorptive medication reported in most studies. The lack of male subjects in the studies could be a rarity of males with osteoporosis requiring antiresorptive medication or a research bias for females. Prevalence studies reporting osteoporosis in > 50 years old was approximately 5-23% females and 1-4% males.7,46,51 Thus, it may be expected that a larger number of females may be available for study recruitment. Furthermore, osteoporosis may diminish osteointegration around implants,52 and increase the risk for implant failure.53
Dental implant failure and ARONJ
ARONJ may not be the etiology of implant failures in all cases. It was reported that implant failures did not result in ARONJ and the failed implants may be replaced successfully. Ten studies18,35,36,39,41-45,48 who reported implant failure (Table 1), 5 reported implant replacement 18,36,42,44,45 resulting in successful osteointegration. There were no reports of ARONJ as an outcome of dental implant surgery nor was it a consequence of implant failure. The failed implants were subsequently replaced with successfully osseointegrated implants, in the patients who did not refuse replacement.18,36, 42,44,45 There were also no healing complications for failed implant removal in patients who refuse implant replacement.18,41,44
The studies (Table 1) that reported implant failure, none encountered ARONJ. The absence of ARONJ in the studies may relate to the route of BP administration and the dosage. The studies in Table 1 were mainly on oral BP. The oral route of BP administration may contribute to a lower risk of ARONJ.
The risk of ARONJ with patients taking IV forms of BPs may be higher. Patients over 70 years old on IV BPs may have a higher risk of osteonecrosis of the jaw.54 This increased risk may be related to patients on IV BPs frequently having accompanying comorbidities, severe osteoporosis, and advanced age. In general, these patients may have an increased risk of ARONJ after any type of oral surgical procedure due to the severity of existing comorbidities. Therefore, implant clinicians need to fully understand the higher risk of ARONJ to these patients, and proceed with caution, or avoid implant surgeries in these IV BP patients.
Effects of BPs on pre-existing dental implants
Patients commencing BPs may be at risk for osseointegration failure of pre-existing dental implants (Table 2). BPs causing failure of pre-existing dental implants may be less common as it has only been reported in some case reports.55-60 These dental implant failures were reported as early as 11 months after starting BP treatment to 4 yrs after. These failed dental implants have been reported to be in function from 2 years to 11 years before disintegration. Patients in these case reports were older than 54 years and the majority were on IV BPs for cancers, while others were on oral BPs for osteoporosis. It is also possible that the reported peri-implantitis in some of these patients could be a precipitating factor for the development of ARONJ.
Table 2: ARONJ in previous implants after initiation of BP treatment (case reports)
| Study | Age (Sex) | Indications | Medications | Time from BP initiation to ARONJ | Duration of implant function | Periodontal condition | Management |
| Favia et al. 201555 | 66 yrs (F) | Breast cancer with metastasis | IV Zoledronate | 4 yrs | 5yrs | NR | Antibiotics Partial mandibular resection |
| Junquera et al. 201156 | 59 yrs (M) | Multiple myeloma | IV Zoledronate | 11 mos | 2 yrs | Healthy | Antibiotics CHX rinse Implant removed |
| Marín-Fernández 62 yrs (F) Breast cancer IV Zoledronate 1 yr 6 yrs et al. 201560 | 62 yrs (F) | Breast cancer with metastasis | IV Zoledronate | 1 yr | 6 yrs | Peri-implantitis | Antibiotics CHX rinse Subtotal maxillectomy |
| Seki et al. 202158 | 73 yrs (F) | Osteoporosis Hypercalcemia | Oral Alendronate | 4 yrs | 10 yrs | Peri-implantitis | Antibiotics Mechanical debridement CHX rinse Maxillary sinus lavage Implant removed |
| Shirota et al. 200959 | 54 yrs (F) | Breast cancer with liver and bone metastasis | IV Pamidronate (current) IV Zoledronate (2005-2006) | 2 yrs | 6 yrs | NR | Antibiotics Hyperbaric oxygen Sequestrectomy |
| Yuan et al. 201260 | 69 yrs (M) | Osteoporosis | Oral Risedronate (2005-2007) Oral Alendronate (2007) | 3 yrs | 11 yrs | Peri-implantitis | Antibiotics Mechanical debridement CHX rinse Implant removed |
Management of implant-related ARONJ
ARONJ may have permanent and severe repercussions for patients such as loss of the maxilla or mandible. ARONJ in previously placed dental implants after starting BP treatment can be treated conservatively with antibiotics, antiseptic mouthrinses, mechanical debridement, and removal of the failed dental implant (Table 2). In more severe cases of ARONJ, subtotal maxillectomy, partial mandibular rection, or sequestrectomy may be needed.
Prevention of ARONJ
ARONJ has been associated with severe infections involving Actinomycetes.61 Thus, it is crucial to lower oral bacterial load with proper oral hygiene in patients on BP needing dental implant surgery. Thus, patients needing dental implants that are periodontally healthy or periodontally stable after periodontal therapy, may contribute to the lower implant failure rates and no ARONJ (Table 1).
Conclusion
Oral or IV antiresorptive medications may impair the integration of dental implants and reduce implant survival. Implant failures in patients on antiresorptive medication do not always lead to osteonecrosis, and failed implants may be replaced with some success. In addition, the commencement of antiresorptive medication may increase the risk of failure in pre-existing and successfully integrated dental implants. Thus, dental implants in patients on antiresorptive medications or commencing antiresorptive medications need to be more meticulously maintained to increase dental implant longevity. Due to the limitations of the available data, larger sample sizes and longer patient follow-ups may be required for more definitive conclusions.
Oral Health welcomes this original article.
References
- Adell R, Lekholm U, Rockler B, Branemark PI. A 15-year study of osseointegrated implants in the treatment of the edentulous jaw. Int J Oral Surg 1981;10(6):387-416.
- Albrektsson T, Dahl E, Enbom L, et al. Osseointegrated oral implants. A Swedish multicenter study of 8139 consecutively inserted Nobelpharma implants. J Periodontol 1988;59(5):287-96.
- Branemark PI, Svensson B, van Steenberghe D. Ten-year survival rates of fixed prostheses on four or six implants ad modum Branemark in full edentulism. Clin Oral Implants Res 1995;6(4):227-31.
- Ekelund JA, Lindquist LW, Carlsson GE, Jemt T. Implant treatment in the edentulous mandible: a prospective study on Branemark system implants over more than 20 years. Int J Prosthodont 2003;16(6):602-8.
- Jemt T, Johansson J. Implant treatment in the edentulous maxillae: a 15-year follow-up study on 76 consecutive patients provided with fixed prostheses. Clin Implant Dent Relat Res 2006;8(2):61-9.
- Lindquist LW, Carlsson GE, Jemt T. A prospective 15-year followup study of mandibular fixed prostheses supported by osseointegrated implants. Clinical results and marginal bone loss. Clin Oral Implants Res 1996;7(4):329-36.
- Pazianas M, Miller P, Blumentals WA, Bernal M, Kothawala P. A review of the literature on osteonecrosis of the jaw in patients with osteoporosis treated with oral bisphosphonates: prevalence, risk factors, and clinical characteristics. Clin Ther 2007;29(8):1548-58.
- Marx RE. Oral and intravenous bisphosphonate-induced osteonecrosis of the jaws : history, etiology, prevention, and treatment. 2nd ed. Chicago: Quintessence Pub. Co., Inc.; 2011.
- Hughes DE, MacDonald BR, Russell RG, Gowen M. Inhibition of osteoclast-like cell formation by bisphosphonates in long-term cultures of human bone marrow. J Clin Invest 1989;83(6):1930-5.
- Hughes DE, Wright KR, Uy HL, et al. Bisphosphonates promote apoptosis in murine osteoclasts in vitro and in vivo. J Bone Miner Res 1995;10(10):1478-87.
- Parfitt AM, Mundy GR, Roodman GD, Hughes DE, Boyce BF. A new model for the regulation of bone resorption, with particular reference to the effects of bisphosphonates. J Bone Miner Res 1996;11(2):150-9.
- Fournier P, Boissier S, Filleur S, et al Bisphosphonates inhibit angiogenesis in vitro and testosterone-stimulated vascular regrowth in the ventral prostate in castrated rats. Cancer Res 2002;62(22):6538-44.
- Mashiba T, Hirano T, Turner CH, et al. Suppressed bone turnover by bisphosphonates increases microdamage accumulation and reduces some biomechanical properties in dog rib. J Bone Miner Res 2000;15(4):613-20.
- Bone Health and Osteoporosis: A Report of the Surgeon General. Rockville (MD); 2004.
- Liberman UA, Weiss SR, Broll J, et al. Effect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosis. The Alendronate Phase III Osteoporosis Treatment Study Group. N Engl J Med 1995;333(22):1437-43.
- Tonino RP, Meunier PJ, Emkey R, et al. Skeletal benefits of alendronate: 7-year treatment of postmenopausal osteoporotic women. Phase III Osteoporosis Treatment Study Group. J Clin Endocrinol Metab 2000;85(9):3109-15.
- Black DM, Thompson DE, Bauer DC, et al. Fracture risk reduction with alendronate in women with osteoporosis: the Fracture Intervention Trial. FIT Research Group. J Clin Endocrinol Metab 2000;85(11):4118-24.
- Zahid TM, Wang BY, Cohen RE. Influence of bisphosphonates on alveolar bone loss around osseointegrated implants. J Oral Implantol 2011;37(3):335-46.
- Suzuki J, Scher E, Ucer C, Lee C. Dental management of patients receiving antiresorptive therapy. Implant Dent Today (London) 2013;7(4):9-16.
- Yamaguchi, A., Kubo, S., Matsunaga, T. & Shibahara, T. (2018) Report by the jaw bone disease project 1: concept, diagnosis and treatment of medication-related osteonecrosis of the jaw. The Shikwa Gakuho, 118, 165-176.
- Delmas PD. Clinical potential of RANKL inhibition for the management of postmenopausal osteoporosis and other metabolic bone diseases. J Clin Densitom 2008;11(2):325-38.
- Cummings SR, San Martin J, McClung MR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med 2009;361(8):756-65.
- Charopoulos I, Orme S, Giannoudis PV. The role and efficacy of denosumab in the treatment of osteoporosis: an update. Expert Opin Drug Saf 2011;10(2):205-17.
- Fizazi K, Carducci M, Smith M, et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castrationresistant prostate cancer: a randomised, double-blind study. Lancet 2011;377(9768):813-22.
- Khosla S, Burr D, Cauley J, et al. Bisphosphonate-associated osteonecrosis of the jaw: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res 2007;22(10):1479-91.
- Siebert T, Jurkovic R, Statelova D, Strecha J. Immediate Implant Placement in a Patient With Osteoporosis Undergoing Bisphosphonate Therapy: 1-Year Preliminary Prospective Study. J Oral Implantol 2015;41 Spec No:360-5.
- Ruggiero SL, Dodson TB, Fantasia J, et al. American Association of Oral and Maxillofacial Surgeons position paper on medicationrelated osteonecrosis of the jaw–2014 update. J Oral Maxillofac Surg 2014;72(10):1938-56.
- Ruggiero SL, Mehrotra B, Rosenberg TJ, Engroff SL. Osteonecrosis of the jaws associated with the use of bisphosphonates: a review of 63 cases. J Oral Maxillofac Surg 2004;62(5):527-34.
- Marx RE, Sawatari Y, Fortin M, Broumand V. Bisphosphonateinduced exposed bone (osteonecrosis/osteopetrosis) of the jaws: risk factors, recognition, prevention, and treatment. J Oral Maxillofac Surg 2005;63(11):1567-75.
- Migliorati CA, Casiglia J, Epstein J, et al. Managing the care of patients with bisphosphonate-associated osteonecrosis: an American Academy of Oral Medicine position paper. J Am Dent Assoc 2005;136(12):1658-68.
- Advisory Task Force on Bisphosphonate-Related Ostenonecrosis of the Jaws AAoO, Maxillofacial S. American Association of Oral and Maxillofacial Surgeons position paper on bisphosphonate-related osteonecrosis of the jaws. J Oral Maxillofac Surg 2007;65(3):369- 76.
- Mavrokokki T, Cheng A, Stein B, Goss A. Nature and frequency of bisphosphonate-associated osteonecrosis of the jaws in Australia. J Oral Maxillofac Surg 2007;65(3):415-23.
- Lazarovici TS, Yahalom R, Taicher S, et al. Bisphosphonate-related osteonecrosis of the jaws: a single-center study of 101 patients. J Oral Maxillofac Surg 2009;67(4):850-5.
- Marx RE, Cillo JE, Jr., Ulloa JJ. Oral bisphosphonate-induced osteonecrosis: risk factors, prediction of risk using serum CTX testing, prevention, and treatment. J Oral Maxillofac Surg 2007;65(12):2397-410.
- Koka S, Babu NM, Norell A. Survival of dental implants in postmenopausal bisphosphonate users. J Prosthodont Res 2010;54(3):108-11.
- Mozzati M, Arata V, Giacomello M, et al. Failure risk estimates after dental implants placement associated with plasma rich in growth factor-Endoret in osteoporotic women under bisphosphonate therapy. J Craniofac Surg 2015;26(3):749-55.
- Lo JC, O’Ryan FS, Gordon NP, et al. Prevalence of osteonecrosis of the jaw in patients with oral bisphosphonate exposure. J Oral Maxillofac Surg 2010;68(2):243-53.
- Jeffcoat MK. Safety of oral bisphosphonates: controlled studies on alveolar bone. Int J Oral Maxillofac Implants 2006;21(3):349-53.
- Tallarico M, Canullo L, Xhanari E, Meloni SM. Dental implants treatment outcomes in patient under active therapy with alendronate: 3-year follow-up results of a multicenter prospective observational study. Clin Oral Implants Res 2016;27(8):943-9.
- Fugazzotto PA, Lightfoot WS, Jaffin R, Kumar A. Implant placement with or without simultaneous tooth extraction in patients taking oral bisphosphonates: postoperative healing, early follow-up, and the incidence of complications in two private practices. J Periodontol 2007;78(9):1664-9.
- Kasai T, Pogrel MA, Hossaini M. The prognosis for dental implants placed in patients taking oral bisphosphonates. J Calif Dent Assoc 2009;37(1):39-42.
- Bell BM, Bell RE. Oral bisphosphonates and dental implants: a retrospective study. J Oral Maxillofac Surg 2008;66(5):1022-4.
- Memon S, Weltman RL, Katancik JA. Oral bisphosphonates: early endosseous dental implant success and crestal bone changes. A retrospective study. Int J Oral Maxillofac Implants 2012;27(5):1216-22.
- Grant BT, Amenedo C, Freeman K, Kraut RA. Outcomes of placing dental implants in patients taking oral bisphosphonates: a review of 115 cases. J Oral Maxillofac Surg 2008;66(2):223-30.
- Famili P, Quigley S, Mosher T. Survival of dental implants among post-menopausal female dental school patients taking oral bisphosphonates: a retrospective study. Compend Contin Educ Dent 2011;32(6):E106-9.
- Al-Sabbagh M, Robinson FG, Romanos G, Thomas MV. Osteoporosis and bisphosphonate-related osteonecrosis in a dental school implant patient population. Implant Dent 2015;24(3):328-32.
- Martin DC, O’Ryan FS, Indresano AT, et al. Characteristics of implant failures in patients with a history of oral bisphosphonate therapy. J Oral Maxillofac Surg 2010;68(3):508-14.
- Yajima N, Munakata M, Fuchigami K, Sanda M, Kasugai S. Influence of Bisphosphonates on Implant Failure Rates and Characteristics of Postmenopausal Woman Mandibular Jawbone. J Oral Implantol 2017;43(5):345-49.
- Chen H, Liu N, Xu X, Qu X, Lu E. Smoking, radiotherapy, diabetes and osteoporosis as risk factors for dental implant failure: a metaanalysis. PLoS One 2013;8(8):e71955.
- Yip JK, Borrell LN, Cho SC, Francisco H, Tarnow DP. Association between oral bisphosphonate use and dental implant failure among middle-aged women. J Clin Periodontol 2012;39(4):408- 14.
- Becker W, Hujoel PP, Becker BE, Willingham H. Osteoporosis and implant failure: an exploratory case-control study. J Periodontol 2000;71(4):625-31.
- Tsolaki IN, Madianos PN, Vrotsos JA. Outcomes of dental implants in osteoporotic patients. A literature review. J Prosthodont 2009;18(4):309-23.
- Alsaadi G, Quirynen M, Komarek A, van Steenberghe D. Impact of local and systemic factors on the incidence of oral implant failures, up to abutment connection. J Clin Periodontol 2007;34(7):610-7.
- Lee CYS, Suzuki JB. Medication-related osteonecrosis of the jaws from once per year intravenous Zoledronic acid (Reclast): report of 4 cases. Implant Dentistry 2015;24(2):227-31.
- Favia G, Tempesta A, Limongelli L, et al. Metastatic Breast Cancer in Medication-Related Osteonecrosis Around Mandibular Implants. Am J Case Rep 2015;16:621-6.
- Junquera L, Gallego L, Pelaz A. Multiple myeloma and bisphosphonate-related osteonecrosis of the mandible associated with dental implants. Case Rep Dent 2011;2011:568246.
- Marin-Fernandez AB, Garcia Medina B, Aguilar-Salvatierra A, Jimenez-Burkhardt A, Gomez-Moreno G. Jaw osteonecrosis management around a dental implant inserted 2 years before starting treatment with zoledronic acid. J Clin Exp Dent 2015;7(3):e444-6.
- Seki K, Namaki S, Kamimoto A, Hagiwara Y. Medication-Related Osteonecrosis of the Jaw Subsequent to Peri-Implantitis: A Case Report and Literature Review. J Oral Implantol 2021;47(6):502-10.
- Shirota T, Nakamura A, Matsui Y, et al. Bisphosphonate-related osteonecrosis of the jaw around dental implants in the maxilla: report of a case. Clin Oral Implants Res 2009;20(12):1402-8.
- Yuan K, Chen KC, Chan YJ, et al. Dental implant failure associated with bacterial infection and long-term bisphosphonate usage: a case report. Implant Dent 2012;21(1):3-7.
- Lee CYS, Pien FD, Suzuki JB. Identification and treatment of bisphosphonate-associated actinomycotic osteonecrosis of the jaw. Implant Dentistry 2011;20(5):331-36.
About the Authors

Dr. Miriam Ting, DMD (Temple University, magna cum laude), BDS (Singapore), Cert. Advanced Periodontology and MS (Craniofacial Biology) at USC. Diplomate, American Board of Periodontology and ICOI. Dr. Ting is founder and director, Think Dental Learning Institute and Magnifico Oral Health Foundation and practises at Think Oral Implants and Periodontics in Paoli, PA.

Dr. Jon B. Suzuki, Clinical Professor, University of Maryland, Baltimore, MD, USA. Clinical Professor, University of , Seattle, WA, USA. Clinical Professor, Nova-Southeastern University, Ft. Lauderdale, FL USA. Professor Emeritus, Temple University, Philadelphia, PA, USA.









