
Abstract
The use of laser therapy has increased in healthcare professions due to its expanding range of applications. After a case in which an increase in keratinized tissue width (KTW) was noted post laser assisted frenectomy, a systematic review of the literature was completed. Within the limitations of this review, there is growing evidence to support laser therapy to increase keratinized tissue width by up to 4.9mm with improved healing, soft tissue stability and improved aesthetics.
Keratinized tissue width (KTW) is a measure of the distance between the free gingival margin and the mucogingival junction,1 and is important for maintaining oral health. A lack of KTW can lead to recession and inflammation,2 because keratinized tissue acts as a barrier against masticatory and external trauma, as well as providing resistance to biofilm.3 According to Cortellini, 2mm of keratinized tissue and 1mm of attached gingiva is recommended to maintain periodontal health in the absence of optimal plaque control.2
The traditional method of increasing KTW is with soft tissue augmentation procedures with the most common being the free gingival graft, as more KTW can be obtained when compared with alternative approaches.4 There have also been case reports that have shown increased keratinized tissue following modified apically repositioned flap procedures.5
A laser therapy, known as laser ablation, is a widely used method for rejuvenating facial skin and enhancing its appearance. It is accomplished by removing the outer layer of skin (epidermis) and heating the deeper layer (dermis) to stimulate collagen growth, resulting in firmer skin. It also leads to a smoother and tighter new layer of skin, because neocollagenesis, neoelastogenesis and fibroblast remodelling occurs.6
There are currently four recognised lasers in dentistry that include: diode (wavelength: 810-980nm), CO2 (wavelength: 10600nm), erbium yttrium aluminium garnet (Er:YAG) (wavelength: 2940nm) and neodymium yttrium aluminium garnet (Nd:YAG) (wavelength: 1064nm).7 Lasers have varying depths of tissue penetration with diodes and Nd:YAG lasers penetrating the deeper dermal layers, whereas the Er:YAG and CO2 lasers remain at the superficial epidermis layer.8 The spot size for lasers is measured using cm2 and the power is quoted in Watts or Joules, which can be adjusted; wavelength is the distance in which a wave repeats and this is usually fixed.9 Lasers can either be a continuous wave (CW) or pulsed high energy, with the CO2 laser exhibiting the highest CW power.10 The CW emits a continuous beam of average to low power in comparison to the pulsed, short high energy beam that last less than 0.25 seconds.11 From a cross sectional study in 2022, 86% of post-graduate periodontal programmes in North America use lasers; of these, 89% use diode lasers in their training.12
Below is a case report documenting an increase in KTW following a laser assisted frenectomy and a systematic review of the supporting literature on the subject.
Case Report
The frenectomy procedure (Fig. 1-3) was completed using ½ carpule of 4% articaine 1:200,000 with the SiroLaser Blue (Dentsply Sirona) at a wavelength of 445nm and at 2.0 W of power, on the continuous wave (CW) setting using the Siro Laser Easy tip 200. The procedure was completed using an ablation technique with the tip moving in a cross-hatched direction. The technique was completed in under 5 minutes and healing occurred by secondary intention. There were no reports of pain experienced by the patient during or after the procedure.
Although there was small evidence of carbonisation (burning/blackening) at the mesial and distal margins, that can delay healing (Fig. 4), the desired outcome was achieved by secondary intention healing. (Fig. 5) At the 4-month post-operative appointment, the healing that had occurred was pleasing to both the patient and clinician, with an increase in KTW by 2mm. (Fig. 6)
Fig. 1
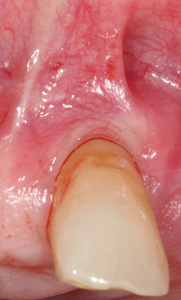
Fig. 2
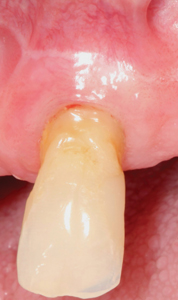
Fig. 3

Fig. 4
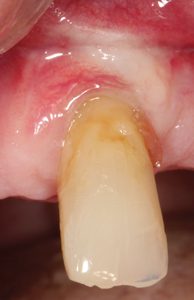
Fig. 5

Fig. 6
Review
Laser therapy has become increasingly popular for various soft tissue procedures in dentistry, such as mucogingival surgeries, open flap debridement, proximal wedge surgeries, second stage exposure of dental implants, gingival depigmentation, and the treatment of peri-implantitis.13
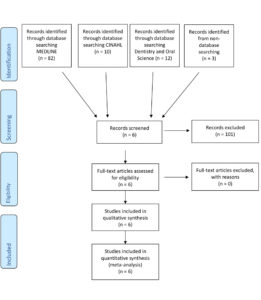
A total of six papers met the inclusion criteria following searches in Medline, CINAHL and Dentistry and Oral Science conducted between September 12th, 2022, and October 12th, 2022. (Fig. 6)
Figure 6 is a PRISMA figure that has been adapted from the preferred reporting of systematic reviews.14
The findings from a total of 138 sites on 88 patients have been summarized in Table 1. These studies reported an increase in KTW ranged from 0.58mm to 4.90mm. Follow-up periods were from 1 week to 12 months, with the greatest KTW increase occurring within the first 3 months following treatment. At 2 months, the pilot prospective study reported an increase in KTW by 1.9mm18 and at 6 months, an increase of 1.44mm was identified by Bhardwaj17 et al. The biggest increase was reported by Uraz et. al at 3 months with an increase of 4.9mm.20 The single case by Benjamin noted an increase of more than 3mm at the 12 month follow-up,16 whilst the split-mouth randomized controlled pilot study reported an increase of 0.58mm.19
Table 1: The findings from 6 papers and a total of 138 sites on 88 patients have been summarized.
| Author | Type of study Reported procedure | Number of patients (sites if stated as different to number of patients) | Length of study (follow-up intervals) | Type of laser | Findings (increase in KTW following laser therapy from baseline) at reported time intervals (mm) |
| Zeinoun et al15 | Case study Apically positioned flap | 19 | 12 months (3 months, 6 months, 12 months) | CO2 laser (2W beam diameter at focal distance; 0.3mm delivery energy density per second = 2829 J/cm2 and wavelength 10600 nm) | 3 months: 3.14 6 months: 3.05 12 months: 2.70 |
| Benjamin16 | Case study Gingival augmentation | 1 | 12 months | Diode laser (3W CW mode, wavelength 970 nm) | 12 months: >3 |
| Bhardwaj et al17 | Case study Periodontal flap surgery with vestibular deepening | 16 (74 sites) | 6 months (clinical parameters were only measured at 6 months) | Diode laser (0.5-7W CW mode, wavelength 810 nm) | 6 months: 1.44 |
| El Hajj et al18 | Pilot prospective study Gingivoplasty following a connective tissue graft | 5 (20 sites): 10 sites received laser therapy, 10 sites received blade therapy | 2 months (clinical and histological parameters) | Diode laser (15 W pulse mode, 9 Khz frequency wavelength 810 nm) | 2 months: 1.90 |
| Ozturan | Split-mouth randomized cont- rolled pilot study Coronally advan-ced flap adjunct with low intensity laser therapy | 10: 17 sites received laser therapy, 17 received no additional treatment | 1 year ( clinical parameters were recorded at baseline and 1 year) | Diode laser (120 mW CW mode, wavelength 588 nm at 4.0 J/cm2) | 1 year: 0.58 |
| Uraz et al20 | Randomised prospective clinical controlled trial Frenectomy | 36: 20 received laser therapy, 16 received scalpel therapy | 3 months (1 week, 1 month, 3 months) | Diode laser (2.8 W CW mode, wavelength 980 nm) | 1 week: 6.75 1 month: 6.15 3 months: 4.90 |
Zeinoun et al. and Uraz et al. noted the change in KTW was reduced over time. Zienoun et al15 noted a mean KTW increase of 3.14 mm at 3 months which was reduced to 2.7 mm at 12 months, Similarly, Uraz et al reported a reduction of 1.85mm between the immediate post-operative visit and the 3 month follow-up (6.75 to 4.90 mm). An increase in KTW was achieved in all studies, despite heterogeneity of wavelengths (588 nm-10600 nm) and the peak pulse power (0.12-14 watts).
There are significant limitations to the studies. Case reports, by their very descriptive nature, are highly biased as the implicit and unconscious biases of the researcher are likely to affect reported results.21 Sample sizes are generally small, limiting generalizability. In addition, there is no comparison group and results may be difficult to replicate.22 Additional limitations include lack of reporting of potential confounders that could affect the results. For example smoking is known to impair wound healing.23 The smoking status of participants was not documented in the clinical trial by Uraz et al.20 or by Benjamin.16 One study gave their patients antibiotics and analgesics following treatment,15 which could affect healing. In addition, none of the studies included a follow-up period that exceeded 12 months.
Low level laser therapy (LLLT), when used immediately following the coronally advanced flap procedure for root coverage to treat recession, improved clinical outcomes in the long-term.19 A systematic review concluded the key superior clinical outcome with LLLT, was a greater gain in KTW when compared with acellular dermal matrix allografts or emdogain as alternatives to the gold standard, autogenous soft tissue.25 LLLT has been shown to be beneficial in increasing the production of ATP (adenosine triphosphate), which increases metabolism and thus increases the energy available for tissue healing.24
There are certain benefits to the use of lasers which include reduced surgical time and decreased post-operative pain, redness and bleeding. As well, patients have reported a sooner return to normal function (speaking and chewing).26 An increase in KTW can improve the overall health of the gingiva and reduce the risk of infection, inflammation, and other complications.
There is confirmed improved histological healing, albeit on rat models, with the use of the laser compared with the scalpel; this includes reduced wound contraction due to fewer myofibroblasts and delayed epithelial regeneration.27,28 In human histological samples, laser gingivoplasty increased KTW compared to blade gingivoplasty as the procedure led to more pronounced parakeratinization and rete pegs (finger-like epithelial projections, formed during epithelial development or the wound healing process; they extend into connective tissue to provide better mechanical resistance and nutritional supply).18 A possible explanation is that laser therapy may provide more time for keratinocytes, which are key for healing, to migrate from the basal membrane to the surface.
The literature in this review has presented the use of CO2 and diode lasers to increase KTW. Compared to other lasers, the diode laser is smaller, more portable, the setup time is minimal, and the associated costs are lower.20 Costs and limited training, especially with the CO2 laser, are the most commonly quoted barriers for the lack of laser usage, even with reported higher patient satisfaction.29 Due to the low level of evidence and heterogeneity of the lasers available, the optimal frequency and wavelength cannot be suggested.
Conclusion
Within the limitations of this review, there is growing evidence to support laser therapy to increase keratinized tissue width by up to 4.9mm with improved healing.
Future research
To strengthen the level of evidence to support the use of lasers in increasing KTW, long-term (>12 months) studies and comparison of laser therapy to conventional techniques, should be conducted through high quality randomized controlled trials.
Oral Health welcomes this original article.
References
- Jepsen, S., et al., Periodontal manifestations of systemic diseases and developmental and acquired conditions: Consensus report of workgroup 3 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J Periodontol, 2018. 89 Suppl 1: p. S237-S248.
- Cortellini, P. and N.F. Bissada, Mucogingival conditions in the natural dentition: Narrative review, case definitions, and diagnostic considerations. J Periodontol, 2018. 89 Suppl 1: p. S204-S213.
- Groeger, S. and J. Meyle, Oral Mucosal Epithelial Cells. Front Immunol, 2019. 10: p. 208.
- Dragan, I.F., et al., Clinical Outcomes of Comparing Soft Tissue Alternatives to Free Gingival Graft: A Systematic Review and Meta-Analysis. J Evid Based Dent Pract, 2017. 17(4): p. 370-380.e3.
- Carnio, J. and P.D. Miller, Increasing the amount of attached gingiva using a modified apically repositioned flap. J Periodontol, 1999. 70(9): p. 1110-7.
- Chen, S.X., et al., Review of Lasers and Energy-Based Devices for Skin Rejuvenation and Scar Treatment With Histologic Correlations. Dermatol Surg, 2022. 48(4): p. 441-448.
- Santonocito, S., et al., Impact of Laser Therapy on Periodontal and Peri-Implant Diseases. Photobiomodul Photomed Laser Surg, 2022. 40(7): p. 454-462.
- Shokrollahi, K., E. Raymond, and M.S.C. Murison, Lasers: principles and surgical applications. The Journal of Surgery, 2004. 2(1): p. 28-34.
- Parker, S., Verifiable CPD paper: introduction, history of lasers and laser light production. Br Dent J, 2007. 202(1): p. 21-31.
- Luk, K., et al., Use of carbon dioxide lasers in dentistry. Lasers in Dental Science, 2019. 3(1): p. 1-9.
- Welch, A.J., J.H. Torres, and W.F. Cheong, Laser physics and laser-tissue interaction. Tex Heart Inst J, 1989. 16(3): p. 141-9.
- Paes Batista da SIlva, A., et al., Status of North American Graduate Programs in Periodontics Providing Laser Education and Clinical Training: A Cross-Sectional Survey. Dent J (Basel), 2022. 10(7).
- Matthews, D.C., Seeing the Light-The Truth about Soft Tissue Lasers and Nonsurgical Periodontal Therapy. Journal of the Canadian Dental Association, 2010. 76(2).
- Moher, D., et al., Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ, 2009. 339: p. b2535.
- Zeinoun, T., et al., CO. Laser Ther, 2017. 26(2): p. 121-127.
- Benjamin, S. Gingival Augmentation: High Powered 970nm Diode Laser. 2017 [cited 2017 June 2017].
- Bhardwaj, A., et al., Periodontal Flap Surgery along with Vestibular Deepening with Diode Laser to Increase Attached Gingiva in Lower Anterior Teeth: A Prospective Clinical Study. J Nat Sci Biol Med, 2018. 9(1): p. 72-76.
- El Hajj, F.A., et al., Changes in Keratinized Tissue Width Following Connective Tissue Grafts and Diode Laser- vs Blade-Gingivoplasty. Int J Periodontics Restorative Dent, 2019. 39(2): p. 279-288.
- Ozturan, S., et al., Coronally advanced flap adjunct with low intensity laser therapy: a randomized controlled clinical pilot study. J Clin Periodontol, 2011. 38(11): p. 1055-62.
- Uraz, A., et al., Patient perceptions and clinical efficacy of labial frenectomies using diode laser versus conventional techniques. J Stomatol Oral Maxillofac Surg, 2018. 119(3): p. 182-186.
- Nissen, T. and R. Wynn, The clinical case report: a review of its merits and limitations. BMC Res Notes, 2014. 7: p. 264.
- Warden, G., Definitions of bias in clinical research. Methods Mol Biol, 2015. 1281: p. 31-48.
- Johnson, G.K. and J.M. Guthmiller, The impact of cigarette smoking on periodontal disease and treatment. Periodontol 2000, 2007. 44: p. 178-94.
- Goldstep, F., Diode Lasers for Periodontal Treatment: The Story Continues. Dental Town, 2010. 11:p. 66-69.
- Graziani, F., et al., Efficacy of periodontal plastic procedures in the treatment of multiple gingival recessions. J Clin Periodontol, 2014. 41 Suppl 15: p. S63-76.
- Gurumoorthy Kaarthikeyan, M., et al., Pain assessment using a visual analog scale in patients undergoing gingival depigmentation by scalpel and 970-nm diode laser surgery. J Laser Dent, 2012. 20: p. 20-3.
- D’Arcangelo, C., et al., A preliminary study of healing of diode laser versus scalpel incisions in rat oral tissue: a comparison of clinical, histological, and immunohistochemical results. Oral Surg Oral Med Oral Pathol Oral Radiol Endod, 2007. 103(6): p. 764-73.
- Fisher, S.E., et al., A comparative histological study of wound healing following CO2 laser and conventional surgical excision of canine buccal mucosa. Arch Oral Biol, 1983. 28(4): p. 287-91.
- Hamada, Y., et al., Dental laser training and education in postgraduate periodontics programs in North America. J Dent Educ, 2022. 86(5): p. 517-525.
About the Authors:

Dr. Matthew Morris is a periodontist based in Halifax and practices across Atlantic Canada. He qualified from Dalhousie University in 2023, where he continues to teach part-time. He has authored peer and non-peer reviewed articles.

Dr. Richard Bengt Price is a Prosthodontist who teaches at Dalhousie University. He is actively involved in research and has authored more than 220 peer-reviewed articles on dental resins, curing lights, and occlusion.

Dr. Debora Matthews is Professor and Director of the Masters in Periodontics program at Dalhousie University. She has been awarded over $6M in research funding and published over 90 peer reviewed articles. She is former Director of the NCOHR and former CDAC chair.









