Abstract
Vitamin D (VD) is a fat-soluble hormone involved in bone metabolism and calcium homeostasis. VD can be produced cutaneously by ultraviolet-B (UVB) irradiation or consumed in the diet. VD is essential for proper bone and tooth development, and its deficiency is prevalent worldwide. Through its effects on bone, VD is postulated to accelerate the process of Orthodontic Tooth Movement (OTM). Various animal studies have investigated the effect of local administration of the active form of VD, calcitriol, and observed a significant increase in the rate of OTM than when compared with matched controls. Vitamin D Deficiency (VDD) in humans was not associated with an increased risk of developing External Apical Root Resorption (EARR) after orthodontic treatment. This review will investigate Vitamin D applications in orthodontics and the potential effects of Vitamin D sufficiency and deficiency on oral health.
Keywords
orthodontics, orthodontic tooth movement, vitamin D, vitamin D deficiency, calcitriol
Introduction – Vitamin D physiology and benefits
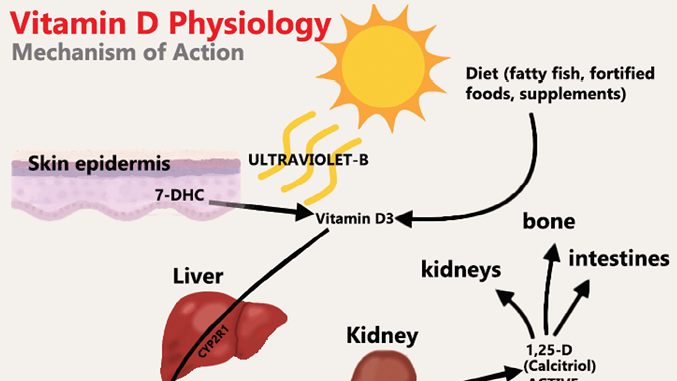
Vitamin D (VD) is the oldest of all hormones.1,2 Known as the “sunshine” vitamin, it is implicated in bone development through calcium and phosphate homeostasis.3 VD is a fat-soluble hormone that comes in 2 main forms or vitamers that differ in their side chains: a plant-based form called ergocalciferol or Vitamin D2, and an animal-based form called cholecalciferol or Vitamin D3.1,4 Vitamin D3 is formed endogenously in human skin by Ultraviolet B irradiation at 290–315 nm wavelengths using sunlight retrieved in the summer months.4 Vitamin D3 is also exogenously obtained in the diet through consumption of fatty fish, supplements, and fortified foods.3,4 Vitamin D3 undergoes two rounds of hydroxylation before achieving its active metabolite form, calcitriol.1,4 Vitamin D3 binds to Vitamin D-binding proteins and, to a lower affinity, albumin in the blood.1,2,4 Ingested Vitamin D3 is first metabolized in the liver for the first round of hydroxylation to become 25-hydroxyvitamin D3 (25-D), then in the kidney for a second round of hydroxylation to become 1α,25-dihydroxyvitamin D3 (1,25-D), or calcitriol (Fig. 1).1,4
Fig. 1
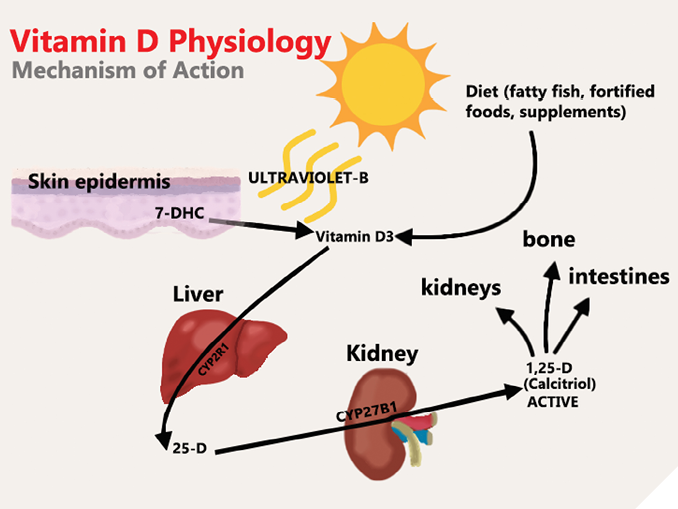
Calcitriol, the active metabolite of VD, is a lipid-soluble hormone that acts on the vitamin D receptor (VDR) which is found inside of nuclei of various cells throughout the body, including cells of the immune system.3 The VDR is a transcription factor that can cause gene regulatory effects when stimulated by calcitriol.3 The diverse range of cell types that the VDR is found in alludes to the versatility of Vitamin D in the human body.3 Most notably, the VDR is found in cells of the intestines, kidneys and bone.4 The stimulation of the VDR by calcitriol in these sites allows for calcium reabsorption in the kidneys, absorption of calcium in the intestines and resorption of bone to mobilize calcium stores.4 For example, the binding of calcitriol to the VDR in the intestines creates calcium binding proteins and channels which increases calcium absorption into the bloodstream.4,5 Calcitriol is in a feedback loop with Parathyroid hormone (PTH), and together control calcium and phosphate levels in the blood.4,6
The most understood benefits of Vitamin D remain to be related to calcium homeostasis and bone development, but the widespread expression of the VDR at different tissues and cells in the body provides Vitamin D with benefits that go beyond bone homeostasis.3,7 For example, the VDR is expressed in human muscle tissue.8 Muscle strength and balance increases with Vitamin D supplementation, and is attributed to a direct genomic effect that stimulates de novo protein synthesis in the muscle, and a non-genomic effect that causes transport of calcium into the muscle cell which facilitates contraction.8 VDR is also expressed in adipocytes, with VD levels in the blood being inversely associated with hepatic and visceral fat accumulation.9 However, in a randomized controlled trial, there was no significant difference in fat accumulation, body composition, blood pressure or insulin resistance when obese individuals were supplemented with VD.9 The data regarding the benefit of VD in Type 2 diabetes, metabolic diseases, and obesity remains inconsistent.9,10 VD is also involved in modulation of the immune system as the VDR is expressed on B and T lymphocytes, monocytes, and macrophages.3 VD works on the innate, and adaptive immune system in various ways that are well documented, and benefits have been linked to prevention against autoimmunity.3,6,11 Most recently, VD supplementation has been suggested to strengthen the immune system as a preventive measure against COVID-19.6
The natural occurring mineral in human bones and teeth is calcium hydroxyapatite, with a formula of Ca₁₀(PO₄)₆(OH)₂.12,13 VD is involved in the regulation of calcium and phosphate ions which are major components of hydroxyapatite, and therefore, VD is naturally involved in the development of bones and teeth.12,13 In children with rickets, the dentition will also undergo developmental changes in what is referred to as the “rachitic tooth”, which increases the incidence of dental caries in those affected teeth.12,14 Vitamin D deficiency remains a high risk factor for osteoporosis, which is associated with progression of periodontitis and periodontal bone loss.14 Moreover, VD may help reduce gingival inflammation by increasing autophagy of P. gingivalis, while decreasing the expression of inflammatory mediators.15
The importance of VD in orthodontics is directly related to the process of orthodontic tooth movement (OTM). The VDR is present on osteoblasts, osteoclasts, and osteoclast precursors.16 OTM is a biological response to prolonged controlled force application on a tooth that causes alveolar bone remodeling.15,16,17 This process involves bone resorption on the pressure side through osteoclastic activity, and bone apposition on the tension side through osteoblastic activity.15,16 VD is capable of inducing osteoclast differentiation and promoting bone resorption.18,19 Recent evidence indicates that VD has the potential to increase the rate of OTM, and promote stability after orthodontic treatment without the added risk of external apical root resorption.17,18,20
Vitamin D deficiency
Vitamin D deficiency (VDD) is an issue of global concern and is associated with a number of diseases including all-cause mortality.21 With this growing concern, an increasing amount of research has been conducted in the past decade to define VDD and accurately understand its consequences.21 VDD guidelines have been proposed by the Endocrine Society Task force in the United States, who have defined VDD as 25-D serum levels that are below 20 ng/ml (50 nmol/liter) and severe VDD to be below 12 ng/ml(30 nmol/liter).22 The current literature on the benefit of VD supplementation is controversial, with the benefits being most pronounced in Vitamin D deficient populations.21,23
VDD can be caused by many acquired and hereditary conditions.24 Most commonly, VDD is caused by a lack of sun exposure to the skin, specifically UVB rays.2,24,25,26 Other reasons for deficiency include inadequate dietary intake of VD.15,21,26 VD status can also be affected by skin pigmentation, age, season, the use of sunscreen lotions, latitude, and altitude.2,4,18,25,26,27,28 Darker skin pigmentation attenuates UVB radiation which lowers the ability to initiate cutaneous production of vitamin D3.28 Moreover, the increased solar zenith angle in countries of higher latitude causes more atmospheric attenuation of solar UVB during the winter, hence less UVB skin exposure throughout the year.28,29 Indoor occupancy is also inadequate to initiate cutaneous production of Vitamin D3. Sunlight entering indoor areas through standard glass windows does not contain UVB needed for Vitamin D production due to the presence of UV filters that differentially block UVA rays and completely block UVB rays from penetrating.30
Severe VDD affects systemic health and oral health.15,24 Most notably, severe VDD causes muscle weakness, osteomalacia in adults, rickets in children, and increases the risk of hip fractures.24,25 Severe VDD causes an increase in Parathyroid Hormone (PTH) which helps resorb bone and reduce bone density, increasing the risk of osteoporosis.4 These changes in PTH and bone density affect the alveolar bone and may impact orthodontic tooth movement.31,32
Prevalence of Vitamin D deficiency
The prevalence of VDD is obtained through national surveys that are standardized to allow the data to be compared across countries and regions.33 It is estimated that more than 1 billion people have VDD worldwide.24 In India alone, it is estimated that close to 490 million people are Vitamin D deficient.33 According to the Endocrine Society Task force guidelines, epidemiological data revealed the prevalence of VDD to be 24% (U.S), 37% (Canada), and 40% (Europe) , while severe VDD was 5.9% (U.S), 7.4% (Canada), and 13% (Europe).26,27,34 That amounts to a crude estimate of 120 million individuals severely VD deficient in the United States, Canada and Europe.33 As concerning as these estimates are, a systematic review of lower-middle income countries (LMICs) revealed an extremely high prevalence of VDD.33,35 Although the data is not standardized, the countries with the highest prevalence of severe VDD are India, Tunisia, Mongolia, Pakistan, and Afghanistan.33,35
The World Health Organization-Food and Agriculture Organization (WHO-FAO) recommends three methods to reduce the incidence of micronutrient malnutrition and VDD. These include (i) consuming a diverse array of food, (ii) food fortification, and (iii) supplementation.33,36 Although increased sun exposure during the summer months is known to increase Vitamin D serum levels, public guidelines cannot recommend increased sun exposure as a treatment strategy due to the increased risk of skin cancer.33 Studies suggest that daily consumption of 20 ug (800 IU) of Vitamin D supplements can maintain 25-D serum levels above 20 ng/mL, considered to be the VDD threshold.21,22 There is no international consensus on the safe upper limit of daily Vitamin D consumption, but most countries recommend the daily safe upper limit to be 50 ug (2000 IU).21,37
Orthodontic tooth movement and Vitamin D
The process of orthodontic tooth movement (OTM) is a biological response to controlled mechanical forces applied onto the teeth that causes alveolar bone remodeling.16,38,39 The average orthodontic active treatment requires 18-24 months to complete.38 Due to the presence of time-dependent side effects of orthodontic treatment such as root resorption and oral hygiene related-problems, it is beneficial to investigate options to facilitate orthodontic treatment by accelerating the rate of OTM.38,40,41 OTM is performed when the applied mechanical force causes subsequent fluid movement in the periodontal ligament (PDL) space which ultimately cause disruption of the PDL cells and activation of a multitude of cell-signaling pathways.16,42 Eventually, PDL turnover along with bone resorption and deposition will occur to accommodate for the movement of teeth.42
Researchers have investigated the use of a variety of pharmacological agents that exhibit the ability to alter the biological response of OTM.39,42 Increasing tissue concentration of the active form of Vitamin D, 1,25-D or calcitriol, has been investigated in multiple animal studies as a modality to increase the rate of OTM and enhance stability after orthodontic treatment.17,43,44,45,46,47 Due to the presence of the VDR on osteoblasts, osteoclast precursors, and active osteoclasts, calcitriol can modulate bone turn-over and potentially expedite orthodontic treatment by increasing the rate of bone resorption.16,17,38 Table 1 summarizes the main animal studies that established the effect of calcitriol local administration on OTM (See Table 1).
Table 1: Summarizing the main animal studies on Vitamin D local administration and its effect on OTM
| Study | Animal Model | Sample Size Exp. | Control | Experimental Group Method | Control Group Method | Results | Notes |
| Kale et al (45), 2004 | Male Sprague-Dawlev rats | 16 | 21 | Group 4: VD/(in DMSO) injections + appliance Group 5: PGE2 (In Lidocaine) injections + appliance | Group 1: no appliance, no injections Group 2: appliance only Group 3: DMSO only, injections + appliance | – group 4 and 5 had significant increase in OTM, and in number of Howship’s lacunae, osteoblasts, and capillaries. | – (P <0.001) – injections every 3 days |
| Takano-Vamamata et al (46), 1992 | Male Wistar Rats (young=7 weeks) and (mature= 28 weeks) | 10 (pilot)3 23 (main) | 10 (pilot) 17 (main) | Group 2: VD(in PBS) + unactivated appliance Group 4: VD(in PBS) + activated appliance Group 5: VD7 (in PBS) + activated appliance Group young 1: D(in PBS) + activated appliance | Group 1: PBS only + unactivated appliance Ground 3: PBS only + activated appliance Group young 2: PBS only + activated appliance | – after 21 days, all groups with VD injection and activated appliance showed significant increase in OTM when compared to other groups and controls – group 4 showed greater OTM than group 5 | – (P <0.05) – injections every 3 days |
| Collins and Sinclair (17), 1988 | Young cats | 5 (pilot) 5 (main) | 5 (pilot)1 5 (main) | DMSO+VD IL injections + 80-g light wire retraction spring | DMSO IL injections + 80.8 light wire retraction spring | After 21 davs. experimental teeth moved 60% further than matched control – no signs of disease | – Significant – (P <0.05) only after 21 days – weekly iniections |
In 1988, Collins and Sinclair demonstrated that intraligamentous injections of 0.1 mL of calcitriol mixed with dimethylsulfoxide (DMSO) solvent in the distal portion of canines in young cats significantly increased the distance of OTM after 21 days of weekly injections and controlled light wire retraction.17 This study pioneered the investigation of local VD administration to potentially increase the rate of OTM. This experiment used a split-mouth design on five young cats where the experimental side received DMSO and VD injections with orthodontic force while the control side received DMSO injections and orthodontic force alone. The paper noted that the experimental tooth always outdistanced its matched control in each cat and the teeth showed no signs of disease in the PDL or the root surface under histological examination.17
Building onto these findings, in 1992, Takano-Yamamoto et al investigated other factors such as age and magnitude of force applied while concomitantly testing for Vitamin D local injection effects on rate and amount of OTM in Wistar rats.46 After administration of submucosal palatal injections of 20 µL of 10-10 mol/L of calcitriol mixed with phosphate-buffered saline (PBS) solvent every three days, a significant increase in OTM was observed on day 21 when compared to the PBS-only matched controls. The study also investigated the effect of age on VD local injections and OTM. It was discovered that young rats had a shorter lag period (5 days) than older rats did (10 days), but VD local administration eliminated this plateau stage and caused continuous tooth movement in both groups.46 When both mature and young rats injected with calcitriol were compared to matched controlled PBS-only injections, the mature rats experienced a 2.5 fold increase in the amount of OTM compared to the 1.2-fold increase in younger rats alluding to the possibility that local injection of calcitriol may be more effective in older rats due to their increased bone density and reduced rate of bone turnover.46 The paper also noted that the lower VD concentration injection of 10-10 mol/L yielded a greater increase in OTM than 10-8 mol/L due to the lower concentration falling within the rat’s physiological range and being more effective in eliciting an osteoclastic response.46
In 2004, Kawakami and Takano-Yamamoto experimented on young Wistar rats again to demonstrate that 1,25-D local injections have the ability to increase osteoblast-mediated bone apposition after orthodontic treatment.47 Their data found a significant increase in bone formation on the resorbed area of bone following orthodontic force and local injection of 1,25-D when compared to PBS-only injections.47 They believe that local injection of calcitriol can expedite OTM through its osteoclastic activity and induce stability after orthodontic treatment through its osteoblastic activity.47
Also in 2004, Kale et al. tested the local injection of PGE2 and compared it to calcitriol injections.45 They observed that both molecules could significantly increase the rate of OTM by inducing bone resorption through osteoclastic activity.45 However, VD was their selected favorite in accelerating OTM because it generated a significantly greater number of osteoblasts than PGE2 during force application and showed a more balanced effect on both bone formation and resorption.45
Recently in the past decade, an experimental study on pregnant Wistar rats observed similar results using systemic intramuscular injections of Vitamin D.43,44 It was also established that VD was associated with the downregulation of a specific cytokine (HMGB1) found in PDL cells during OTM, which facilitates a microenvironment that favors tooth movement.48
Vitamin D and root resorption
Like many medical procedures, orthodontic treatment comes with a variety of risks including External Apical Root Resorption (EARR).40,49 EARR is a multifactorial undesirable iatrogenic consequence associated with orthodontic treatment and theorized to be caused by excessive osteoclastic activity.49,50 Since VD can act directly on osteoclasts, it is essential to investigate the relationship between Vitamin D and the incidence of EARR after orthodontic treatment.16,38 Tehranchi et al demonstrated that VDD and VD serum levels are not amongst the clinical variables that can contribute to EARR.51 Patients with EARR by the end of orthodontic treatment were not more likely to be VD deficient.51 Another study found that a certain VDR gene polymorphism was weakly associated with protection against EARR after orthodontic treatment.20 In addition, the experimental animal studies discussed earlier noted no histological signs of disease on the root surface in the teeth adjacent to the injection sites.17,45,46 Therefore, there is currently no evidence to suggest that vitamin D local administration or systemic deficiency may increase the risk of developing EARR after orthodontic treatment. However, there is some evidence to suggest that Vitamin D may in fact serve as a protective agent against EARR in certain individuals with the TaqI vitamin D receptor gene polymorphism.20
Conclusion
Vitamin D is a versatile hormone involved in bone mineralization, muscle contraction, and the immune system.3,4,8 Vitamin D deficiency can affect the development of teeth and bone, and is associated with all-cause mortality.12,13,21 The prevalence of severe VDD is crudely estimated to be 120 million in the United States, Canada, and Europe.33 There is potential for patients seeking dental and orthodontic treatment to be VD deficient. VD is capable of acting directly on osteoblasts and osteoclast to expedite the process of Orthodontic Tooth Movement and increase stability after orthodontic treatment.16,17,38 Multiple animal studies have demonstrated that local injections of the active form of VD, calcitriol, were able to accelerate OTM with no histological signs of disease on the root surface.17,45,46 VDD was not associated with the increased risk of External Apical Root Resorption.51
Further research is needed to determine whether systemic human VDD is associated with the rate of OTM. In addition, the local administration of calcitriol should be investigated in human trials to accelerate the rate of OTM. Researchers should understand the optimal dose, frequency, and method of local VD administration in humans to expedite orthodontic treatment and minimize the incidence of time-dependent orthodontic iatrogenic effects. 
Oral Health welcomes this original article.
References
- Bikle DD. Vitamin D: An ancient hormone. Exp Dermatol. 2011;20(1):7–13.
- Holick MF, Chen TC, Lu Z, Sauter E. Vitamin D and skin physiology: A D-lightful story. J Bone Miner Res. 2007;22(SUPPL. 2):28–33.
- Carlberg C. The physiology of vitamin D – far more than calcium and bone. Front Physiol. 2014;5 AUG(September):1–2.
- Lips P. Vitamin D physiology. Prog Biophys Mol Biol. 2006;92(1):4–8.
- Christakos S, Li S, De La Cruz J, Shroyer NF, Criss ZK, Verzi MP, et al. Vitamin D and the intestine: Review and update. J Steroid Biochem Mol Biol. 2020;196(October 2019).
- Grant WB, Lahore H, McDonnell SL, Baggerly CA, French CB, Aliano JL, et al. Evidence that vitamin d supplementation could reduce risk of influenza and covid-19 infections and deaths. Nutrients. 2020;12(4):1–19.
- Campolina-Silva GH, Maria BT, Mahecha GAB, Oliveira CA. Reduced vitamin D receptor (VDR) expression and plasma vitamin D levels are associated with aging-related prostate lesions. Prostate. 2018;78(7):532–46.
- Bischoff-Ferrari HA. Relevance of vitamin D in muscle health. Rev Endocr Metab Disord. 2012;13(1):71–7.
- Wamberg L, Kampmann U, Stødkilde-Jørgensen H, Rejnmark L, Pedersen SB, Richelsen B. Effects of vitamin D supplementation on body fat accumulation, inflammation, and metabolic risk factors in obese adults with low vitamin D levels – Results from a randomized trial. Eur J Intern Med [Internet]. 2013;24(7):644–9. Available from: http://dx.doi.org/10.1016/j.ejim.2013.03.005
- Sacerdote A, Dave P, Lokshin V, Bahtiyar G. Type 2 Diabetes Mellitus, Insulin Resistance, and Vitamin D. Curr Diab Rep. 2019;19(10).
- Prietl B, Treiber G, Pieber TR, Amrein K. Vitamin D and immune function. Nutrients. 2013;5(7):2502–21.
- Foster BL, Nociti FH, Somerman MJ. The rachitic tooth. Endocr Rev. 2014;35(1):1–34.
- Mahdavi R, Belgheisi G, Haghbin-Nazarpak M, Omidi M, Khojasteh A, Solati-Hashjin M. Bone tissue engineering gelatin–hydroxyapatite/graphene oxide scaffolds with the ability to release vitamin D: fabrication, characterization, and in vitro study. J Mater Sci Mater Med [Internet]. 2020;31(11). Available from: http://dx.doi.org/10.1007/s10856-020-06430-5
- Uwitonze AM, Murererehe J, Ineza MC, Harelimana EI, Nsabimana U, Uwambaye P, et al. Effects of vitamin D status on oral health. J Steroid Biochem Mol Biol [Internet]. 2018;175(2016):190–4. Available from: http://dx.doi.org/10.1016/j.jsbmb.2017.01.020
- Botelho J, Machado V, Proença L, Delgado AS, Mendes JJ. Vitamin D deficiency and oral health: A comprehensive review. Nutrients. 2020;12(5).
- Sidhu S. Drug Induced Orthodontic Tooth Movement. J Adv Med Dent Scie Res [Internet]. 2019;7(4):5–7. Available from: www.jamdsr.com
- Collins MK, Sinclair PM. The local use of vitamin D to increase the rate of orthodontic tooth movement. Am J Orthod Dentofac Orthop. 1988;94(4):278–84.
- Almoammar K. Vitamin D and orthodontics: An insight review. Clin Cosmet Investig Dent. 2018;10:165–70.
- Takahashi N, Udagawa N, Suda T. Vitamin D endocrine system and osteoclasts. Bonekey Rep [Internet]. 2014;3(October 2013):1–9. Available from: http://dx.doi.org/10.1038/bonekey.2013.229
- Fontana MLSSN, De Souza CMH, Bernardino JF, Hoette F, Hoette ML, Thum L, et al. Association analysis of clinical aspects and vitamin D receptor gene polymorphism with external apical root resorption in orthodontic patients. Am J Orthod Dentofac Orthop. 2012;142(3):339–47.
- Amrein K, Scherkl M, Hoffmann M, Neuwersch-Sommeregger S, Köstenberger M, Tmava Berisha A, et al. Vitamin D deficiency 2.0: an update on the current status worldwide. Eur J Clin Nutr [Internet]. 2020;74(11):1498–513. Available from: http://dx.doi.org/10.1038/s41430-020-0558-y
- 22. Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, treatment, and prevention of vitamin D deficiency: An endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911–30.
- 23. Giustina A, Adler RA, Binkley N, Bouillon R, Ebeling PR, Lazaretti-Castro M, et al. Controversies in Vitamin D: Summary Statement from an International Conference. J Clin Endocrinol Metab. 2018;104(2):234–40.
- 24. Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–81.
- 25. Holick MF. Deficiency of sunlight and vitamin D. Br Med J. 2008;336:1318–9.
- 26. Schleicher RL, Sternberg MR, Looker AC, Yetley EA, Lacher DA, Sempos CT, et al. National estimates of serum total 25-hydroxyvitamin D and metabolite concentrations measured by liquid chromatography-tandem mass spectrometry in the US population during 2007-2010. J Nutr. 2016;146(5):1051–61.
- 27. Cashman KD, Dowling KG, Škrabáková Z, Gonzalez-Gross M, Valtueña J, De Henauw S, et al. Vitamin D deficiency in Europe: Pandemic? Am J Clin Nutr. 2016;103(4):1033–44.
- 28. Greenfield JA, Park PS, Farahani E, Malik S, Vieth R, McFarlane NA, et al. Solar ultraviolet-B radiation and vitamin D: A cross-sectional population-based study using data from the 2007 to 2009 Canadian Health Measures Survey. BMC Public Health. 2012;12(1).
- 29. El-Nouby Adam M. Effect of The Atmosphere on UVB Radiation Reaching the Earth’s Surface: Dependence on Solar Zenith Angle. Atmos Ocean Sci Lett. 2011;4(3):139–45.
- 30. Duarte I, Rotter A, Malvestiti A, Silva M. The role of glass as a barrier against the transmission of ultraviolet radiation: An experimental study. Photodermatol Photoimmunol Photomed. 2009;25(4):181–4.
- 31. Apostolos I. Tsolakis, Khaldi L, Bitsanis I, Alexandridis C, Αggeliki T, Meropi N. Spyropoulos IAD. The effect of osteopenia on tooth movement in ovariectomized rats. An experimental study Apostolos. J Musculoskelet Neuronal Interact. 2018;18(3):366–74.
- 32. Lee HS, Heo HA, Park SH, Lee W, Pyo SW. Influence of human parathyroid hormone during orthodontic tooth movement and relapse in the osteoporotic rat model: A preliminary study. Orthod Craniofacial Res. 2018;21(3):125–31.
- 33. Cashman KD. Vitamin D Deficiency: Defining, Prevalence, Causes, and Strategies of Addressing. Calcif Tissue Int [Internet]. 2020;106(1):14–29. Available from: https://doi.org/10.1007/s00223-019-00559-4
- 34. Sarafin K, Durazo-Arvizu R, Tian L, Phinney KW, Tai S, Camara JE, et al. Standardizing 25-hydroxyVitamin D values from the Canadian Health Measures Survey. Am J Clin Nutr. 2015;102(5):1044–50.
- 35. Cashman KD, Sheehy T, O’Neill CM. Is vitamin D deficiency a public health concern for low middle income countries? A systematic literature review. Eur J Nutr [Internet]. 2019;58(1):433–53. Available from: http://dx.doi.org/10.1007/s00394-018-1607-3
- 36. Allen L, de Benoist B, Dary O HR. Guidelines on food fortification with micronutrients [Internet]. World Health Organization. 2006. Available from: https://apps.who.int/iris/handle/10665/43412
- 37. Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride [Internet]. Washington (DC): National Academies Press (US); 1997. 448 p. Available from: http://www.nap.edu/catalog/5776.html
- 38. Li Y, Jacox LA, Little SH, Ko CC. Orthodontic tooth movement: The biology and clinical implications. Kaohsiung J Med Sci. 2018;34(4):207–14.
- 39. Kouskoura T, Katsaros C, von Gunten S. The potential use of pharmacological agents to modulate orthodontic tooth movement (OTM). Front Physiol. 2017;8(FEB):1–9.
- 40. Wishney M. Potential risks of orthodontic therapy: a critical review and conceptual framework. Aust Dent J. 2017;62:86–96.
- 41. Huang H, Williams RC, Kyrkanides S. Accelerated orthodontic tooth movement: Molecular mechanisms. Am J Orthod Dentofac Orthop [Internet]. 2014;146(5):620–32. Available from: http://dx.doi.org/10.1016/j.ajodo.2014.07.007
- 42. Bartzela T, Türp JC, Motschall E, Maltha JC. Medication effects on the rate of orthodontic tooth movement: A systematic literature review. Am J Orthod Dentofac Orthop [Internet]. 2009;135(1):16–26. Available from: http://dx.doi.org/10.1016/j.ajodo.2008.08.016
- 43. Megat Badarul Hisham PN, Narmada I, Alida A, Rahmawati D, Nugraha A, Putranti N. Effects of Vitamin D in Alveolar Bone Remodeling on Osteoblast Numbers and Bone Alkaline Phosphatase Expression in Pregnant Rats during Orthodontic Tooth Movement. J Orofac Sci. 2019;11(2):79–83.
- 44. Narmada IB, Husodo KRD, Ardani IGAW, Rahmawati D, Nugraha AP, Iskandar RPD. Effect of vitamin D during orthodontic tooth movement on receptor activator of nuclear factor Kappa-B ligand expression and osteoclast number in pregnant wistar rat (Rattus novergicus). J Krishna Inst Med Sci Univ. 2019;8(1):37–42.
- 45. Kale S, Kocadereli I, Atilla P, Aşan E. Comparison of the effects of 1,25 dihydroxycholecalciferol and prostaglandin E2 on orthodontic tooth movement. Am J Orthod Dentofac Orthop. 2004;125(5):607–14.
- 46. Takano-Yamamoto T, Kawakami M, Yamashiro T. Effect of Age on the Rate of Tooth Movement in Combination with Local Use of 1,25(OH) 2D3 and Mechanical Force in the Rat. J Dent Res. 1992;71(8):1487–92.
- 47. Kawakami M, Takano-Yamamoto T. Local injection of 1,25-dihydroxyvitamin D3 enhanced bone formation for tooth stabilization after experimental tooth movement in rats. J Bone Miner Metab. 2004;22(6):541–6.
- 48. Cui J, Li J, Wang W, Han X, Du J, Sun J, et al. The effect of calcitriol on high mobility group box 1 expression in periodontal ligament cells during orthodontic tooth movement in rats. J Mol Histol. 2016;47(2):221–8.
- 49. Travess H, Roberts-Harry D, Sandy J. Orthodontics. Part 6: Risks in orthodontic treatment. Br Dent J. 2004;196(2):71–7.
- 50. Topkara A, Karaman AI, Kau CH. Apical root resorption caused by orthodontic forces: A brief review and a long-term observation. Eur J Dent. 2012;6(4):445–53.
- 51. Tehranchi A, Sadighnia A, Younessian F, Abdi A, Shirvani A. Correlation of Vitamin D status and orthodontic-induced external apical root resorption. Dent Res J (Isfahan). 2017;14(6):403–11
About the authors

Dr. Abdallah Omar graduated with a Doctor of Dental Surgery from the University of Toronto and completed a General Practice Residency at SickKids Hospital. He practices in Vaughan and Mississauga as a dentist, focusing on pediatric dentistry.

Dr. Aviv Ouanounou is an associate professor at the Faculty of Dentistry, University of Toronto. Dr. Ouanounou is regularly invited to speak at conferences locally, nationally and internationally. He is the recipient of the 2014-2015 Dr. Bruce Hord Master Teacher Award for excellence in teaching and the 2018-2019 National W.W. Wood Teaching Award for Excellence in Dental Education. Dr. Ouanounou maintains a private practice in Toronto. Dr. Ouanounou is the corresponding author of this article and can be reached at aviv.ouanounou@dentistry.utoronto.ca.