Introduction
The use of digital planning in the practice of oral and maxillofacial surgery has afforded clinicians with enhanced treatment planning capabilities through the fabrication of surgical stents, which results in reduced intra-operative time and improved predictability of surgical outcomes. This method of pre-operative planning utilizes digital imaging data to plan surgery and construct the necessary guides for its completion. While commonly employed in the discipline of oral and maxillofacial surgery for traditional orthognathic surgical procedures, this technology can be applied to aid in the treatment of gnathic pathology.
Fibrous dysplasia (FD) is a benign and sporadic process, which is the result of increased but inappropriate cell differentiation. This occurs due to a genetic defect in the GNAS1 gene located on the long arm of chromosome 20, which affects the ability of skeletal stem cells to become normally functioning osteoblasts.1 This results in the replacement of normal bone with an unorganized fibrous tissue matrix and abnormal bone production.1,2 The timing of this mutation during embryogenesis determines the distribution of the disease process. When this mutation occurs late in development, FD tends to affect a single bone most commonly in the craniofacial complex. This is termed monostotic FD. Multiple bones are affected if the mutation occurs at earlier stages of embryogenesis, causing polyostotic FD. In the event that the mutation occurs during the embryological development of the inner cell mass, all three germ layers are affected and is termed McCune-Albright syndrome (MAS). This results in polyostotic FD, café au lait skin pigmentation and a multitude of endocrinopathies including precocious puberty, hyperthyroidism and hypercortisolism.1,2 On occasion, FD can involve extra-gnathic sites such as the ribs, femur and tibia.3 The most striking clinical feature of gnathic FD is unilateral facial asymmetry due to an enlargement of one or both of the jaws. Pain is an atypical finding but when FD involves other bones of the skull, neural foramina may be reduced in caliber resulting in neural deficits such as anosmia, blindness and deafness.4
Patients diagnosed with FD must be reassured that it is a benign process whereby bone has been replaced by fibrous tissue; it is very rarely associated with malignant transformation.5,6 Additionally, patients should understand that this disease is self-limiting and terminates, in most cases, when skeletal maturity is reached.7,8 At this time, patients may opt to undergo cosmetic re-contouring of the affected gnathic structures, which can be aided through the utilization of digital planning.
Report of Case
A 17-year-old male patient initially presented to the Division of Oral and Maxillofacial Surgery at Mount Sinai Hospital (Toronto, ON) for the surgical management of a right-sided facial asymmetry that was associated with mild and transient pain. A biopsy of the right mandible was performed to confirm the diagnosis of FD (Fig. 1) revealing randomly arranged non-contiguous and curvilinear bony trabeculae in a background of fibrous connective tissue, with minimal osteoblastic rimming. The patient was advised to delay surgical treatment until the completion of skeletal maturity, and was followed until the age of 25 when it was determined that there was no further growth of the lesion. At this time, there was an appreciable extra-oral right-sided mandibular enlargement (Figs. 2A-C). Intraorally, there was palpable and firm expansion of the buccal and lingual surfaces of the right mandible with no discernible displacement of the dentition in the region of interest (Fig. 3).
FIGURE 1. Biopsy of right mandible (Decalcified section stained with hematoxylin and eosin stain, magnification x2.5).

FIGURE 2A – F. Clinical photographs. A-C: Pre-operative right lateral and frontal images demonstrating right-sided mandibular asymmetry. D-F, Post-operative right lateral and frontal images demonstrating markedly improved mandibular symmetry.
FIGURE 2A. FIGURE 2B.


FIGURE 2C. FIGURE 2D.
FIGURE 3. Intraoral photograph demonstrating right-sided mandibular enlargement and no gross disturbances of the occlusion.

Pre-operative panoramic and non-contrast enhanced computed tomographic (CT) imaging revealed a relatively well-defined and partially corticated, which extends from the mandibular left first premolar to the right mandibular condyle. The internal structure of this entity was heterogeneous and composed of relatively radiopaque regions possessing a granular bone pattern with several haphazardly distributed, well-defined and partially corticated cyst-like regions. The cortices of the mandible in the region of interest remained intact, but a medial displacement of the right inferior alveolar canal was observed. The imaging findings were consistent with fibrous dysplasia (Figs. 4A & 5A-I). Finally, there was a diffuse heterogeneous uptake of 99m Tc-MDP (Technetium Methyl Diphosphonate) in the single-photon emission computed tomography (SPECT) study throughout the right mandible (Figs 6A-D). These findings suggest the presence of osteoblastic activity, which is consistent with the patient’s known history of fibrous dysplasia. However, no inference can be made with regards to the dimensional stability of this lesion using this imaging modality alone.
FIGURE 4A. & B. Panoramic images. A. Pre-operative, B. Post-operative.
FIGURE 4A.

FIGURE 4B.

FIGURE 5A – I. Selected pre-operative CT images (bone window). A-C: Coronal plane, D-F: Axial plane, G-I: Sagittal plane.
FIGURE 5A. FIGURE 5B. FIGURE 5C.



FIGURE 5D. FIGURE 5E. FIGURE 5F.



FIGURE 5G. FIGURE 5H. FIGURE 5I.



FIGURES 6A-D. Pre-operative SPECT images. A-B: Selected coronal images, C-D: Selected sagittal images.
Pre-operative laboratory tests were within the range of normal and informed consent was obtained for the corticotomy of the right mandible under general anesthesia.
Materials and Methods
Several weeks prior to the procedure, the DICOM (Digital Imaging and Communications in Medicine) files from the pre-operative CT scan were sent for PROPLAN CMF/DePuy Synthes TruMatch CMF Solutions® (Materialise, Plymouth, MI) to plan (Figs. 7 & 8) and fabricate a custom occlusal-based acrylic corticotomy splint (Fig. 9).
FIGURE 7A.-H. Pre-operative three dimension rendering of the mandible – A: Inferior view, B: Right lateral oblique view, E: Frontal view, C: Right lateral view. VSP reduction with the semi-transparent overlay of the pre-operative mandibular anatomy–B: Inferior view, D: Right lateral oblique view, F: Frontal view, G: Right lateral view.
FIGURE 7A. FIGURE 7B.

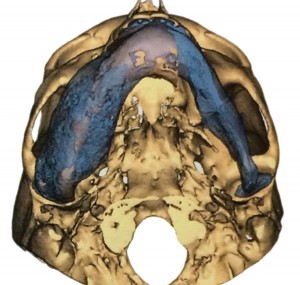
FIGURE 7C. FIGURE 7D.
FIGURE 7E. FIGURE 7F.
FIGURES 8A. & B. Superimposition of VSP reduction and surgical splint with the semi-transparent overlay of the pre-operative mandibular anatomy. A: Right lateral view, B: Frontal view.
FIGURES 9A. & B. Acrylic reduction guide – A: Lateral view, B: Superior view.
FIGURE 9A.

The procedure was performed in hospital under general anesthesia via nasotracheal intubation. Local anesthesia with 2% Xylocaine with 1:100,000 epinephrine (Pfizer, Kirkland, PQ) was delivered via a right and left-sided Gow-Gates block, local infiltration and C1-4 superficial cervical plexus block. Degloving of the mandible with a full-thickness mucoperiosteal flap was extended from the left mandibular canine region to the anterior surface of the right mandibular ramus while careful attention was paid to the integrity of the right mental nerve, which was protected throughout the procedure. The right masseter muscle was stripped from the mandible to allow for passive seating of the surgical splint. An initial gross buccal and inferior border corticotomy of the right mandible was performed using a reciprocating saw so as to allow for the placement of the rigid acrylic splint. Following this, the corticotomy was completed with a barrel bur and a diamond reciprocating rasp under copious saline irrigation until the surgical splint rested passively along the buccal surface of the right mandible with the patient in the pre-operative occlusal relationship. Once completed, the surgical site was irrigated copiously and closed with 3-0 chromic gut sutures (Johnson & Johnson, Guelph, ON) in a continuous fashion followed by the application of a Barton bandage.
FIGURE 10. Intra-operative view demonstrating the passive seating of the acrylic reduction guide.

Results
The patient awoke from general anesthesia, was extubated, admitted to the post-anesthesia care unit and then a surgical ward for overnight observation. The patient was discharged the next day in stable condition with no evidence of intraoral hemorrhage or other complications.
The patient enjoyed an uneventful post-operative course and a markedly improved physical appearance at a six-month post-operative evaluation (Figs. 2D-F). Post-operative
panoramic imaging demonstrates a reduction in the inferior-superior dimension of the right mandible although the abnormal bone pattern in this region that was previously described remains present (Fig. 4B). The patient is now at 24 months post-surgery without any sign of regrowth.
Discussion
Management of patients with large facial asymmetries secondary to a diagnosis of FD involves careful pre-operative planning, and, in this case, the application of digital planning techniques. Unfortunately, the timing, technique and indications for surgical intervention of FD remain controversial.8,9 The primary objectives of surgery for FD are the correction or prevention of functional deficits and the restoration or improvement of aesthetic appearances. FD can have devastating consequences and is associated with recurrence rates of as high as 75% when partial resection is selected.9,10 While the prognosis of monostotic FD is good, as in this case, the patient was informed that despite the fact that he has reached skeletal maturity and the lack of growth of the lesion, there would still be a risk for continued lesional growth and the possible need for further surgical management. The aggressiveness of the surgical approach is largely dependent on the craniofacial region involved, the potential for recurrence given the subtype of FD and the patient’s state of skeletal maturity. Generally, the radical approach is opted for in those regions with the greatest probability of functional impairment with immediate reconstruction to restore function and symmetry.8
To help plan the corticotomy for this case, CT-guided navigation could have been utilized.11 This technique relies on the use of a navigational probe to register the patient’s craniofacial skeleton with the pre-operative CT scan to aid in determining the appropriate reduction in an intra-operative setting. Unfortunately, this requires the use of and access to expensive CT navigation equipment and is subject to positioning and registration errors. This would be especially problematic in regions where the avoidance of vital anatomy is crucial. Furthermore, the registration, and re-registration processes can markedly increase the length of the surgical procedure and requires the patient’s head to be immobilized.
The guidance method chosen for the osseous reduction in this case possessed several advantages over the aforementioned method. First, digital planning reduced the intra-operative time by allowing for a pre-operative surgical simulation where the exact extent of the reduction is decided upon in advance of the procedure. Second, the use of the acrylic reduction guide allowed for the immediate intra-operative targeting of the osseous structures that required reduction. Finally, the addition of the occlusal extension to the stent provided the surgeons with a reliable and consistent manner in which to position and anchor the guide so that the reduction would mirror the surgical plan. The combination of these benefits also provided the patient with a more thorough understanding of the nature and goals of the procedure, thereby improving the process of informed consent. Ultimately, this leads to increased satisfaction with the final functional and aesthetic result, which will significantly impact the patient’s quality of life.OH
Jeffrey W. Chadwick, Discipline of Oral and Maxillofacial
Radiology, University of Toronto, Toronto, Ontario.
Garry Toor, Chief Resident, Discipline of Oral and Maxillofacial Surgery, University of Toronto, Toronto, Ontario.
Marco Caminiti, Assistant Professor, Discipline of Oral and Maxillofacial Surgery, University of Toronto, Toronto, Ontario.
Oral Health welcomes this original article.
References:
1. Akintoye SO, Boyce AM, Collins MT. Dental perspectives in fibrous dysplasia and McCune-Albright syndrome. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;116(3):e149-55.
2. Eversole R, Su L, ElMofty S. Benign fibro-osseous lesions of the craniofacial complex. A review. Head Neck Pathol. 2008;2(3):177–202.
3. McCarthy EF. Fibro-osseous lesions of the maxillofacial bones. Head Neck Pathol. 2013;7(1):5-10.
4. Posnick JC. Fibrous dysplasia of the craniomaxillofacial region: current clinical perspectives. Br J Oral Maxillofac Surg, 1998;36:264–273.
5. Ruggieri P, Sim FH, Bond JR, Unni KK. Malignancies in fibrous dysplasia. Cancer. 1994;73(5):1411-24
6. Hoshi M, Matsumoto S, Manabe J, Tanizawa T, Shigemitsu T, Izawa N, Takeuchi K, Kawaguchi N. Malignant change secondary to fibrous dysplasia. Int J Clin Oncol. 2006;11(3):229-35.
7. DiCaprio MR, Enneking WF. Fibrous Dysplasia: Pathophysiology, Evaluation, and Treatment. J Bone Joint Surg Am. 2005;87(8):1848-64.
8. Ricalde P, Magliocca KR, Lee JS. Craniofacial fibrous dysplasia. Oral Maxillofac Surg Clin North Am. 2012;24(4):427-41
9. Lichtenstein L, Jaffe HL. Fibrous dysplasia of bone. A condition affecting one, several or many bones, the graver cases of which may present abnormal pigmentation of skin, premature sexual development, hyperthyroidism or still other extraskeletal abnormalities. Arch Pathol. 1942;33:777-816.
10. Fattah A, Khechoyan D, Phillips JH, Forrest CR. Paediatric craniofacial fibrous dysplasia: The Hospital for Sick Children experience and treatment philosophy. J Plast Reconstr Aesthet Surg. 2013;66(10):1346-55.
11. Wang X, Lin Y, Yu H, Cheng AH, Sun H, Wang C, Shen G. Image-Guided Navigation in Optimizing Surgical Management of Craniomaxillofacial Fibrous Dysplasia. J Craniofac Surg. 2011;22(5):1552-6.
Follow the Oral Health Group on Facebook, Instagram, Twitter and LinkedIn for the latest updates on news, clinical articles, practice management and more!





























