
Dental Implants have impressive success rates approaching 95-97% and have become a preferred treatment plan for edentulous and partially edentulous dentitions for both patients and clinicians. However, success rates have increasingly been dampened by reports of implant failures with varying degrees of success for clinical rescue. Initial signs and symptoms for implant diseases have now been recognized. Progressive patterns of bone loss around dental implants (Peri-Implantitis) have been identified with accompanying recognized etiologies. Non-surgical and surgical therapies are now published and may be included as potential therapies for implant health and disease levels.
Dr. Branemark introduced “osseointegration” to describe this modality for stable titanium and bone tissue fixation and as a direct bone-to-implant contact without interposed soft tissue.1,2
Dental implant stability and longevity are influenced by factors that affect bone remodelling and bone maintenance at the implant surface.3 Adequate bone thickness and vasculature may be a physiological requirement for obtaining nutrients and appropriate remodelling around the implant. Systemic factors, local factors, implant platform, implant surface characteristics, and prosthetic designs can influence bone and vasculature for osteocyte nutrition and function. The early stage of peri-implant bone healing involves the body’s initial response to foreign materials with protein adsorption, platelet activation, coagulation, and inflammation. The later stage of alveolar bone remodelling is influenced by the functional occlusal loading on opposing dentition after delivery of implant prosthesis.4
Establishing adequate keratinized gingiva height and attachment is also essential for more predictable maintenance, improved aesthetics and protection of the implant and surrounding bone.5,6 A meta-analysis indicated inadequate keratinized mucosa around dental implants is associated with more plaque accumulation, tissue inflammation, mucosal recession, and attachment loss.7
According to the 2017 World Workshop on the Classification of Periodontal and Peri-implant Diseases and Conditions and the 2013 American Academy of Periodontology report, no single diagnostic tool can, with certainty, establish a diagnosis of peri-implantitis. Diagnostic tools that have been mentioned in the reports are the presence of bone loss, radiographic changes in bone levels over time, probing depths, inflammatory status of mucosa, bleeding on probing, suppuration, recession of the mucosal margin, mobility of implant, and possibly bacterial and/or peri-implant crevicular fluid sample size.8,9
Maintenance of bone levels around dental implants is a commonly used criterion for success. Marginal bone level is determined by comparing recent radiographs taken to radiographs taken after loading of the implant prosthesis. Studies have shown that during the first year of functional occlusal loading, marginal bone level changes of 1.0 to 2.0 mm may be expected due to biological bone remodelling around the prosthesis.10,11,13,14,15 Albreksson et al. 1986 indicated that an implant should have less than 0.2 mm vertical bone loss per year after the first year.16,17 Laurell & Lundgren’s 2011 meta-analysis studies have shown that different implant designs and surface characteristics have different annual bone loss rates that do not apply the 0.2 mm per year criteria.18
Greenstein et al. 2019 indicated that desirable probing depths around dental implants are 2.5 mm to 4 mm, but deeper probing depths can be associated with healthy peri-implant mucosa.19 According to the 2017 World Workshop, it is not possible to define a range of probing depths compatible with health20 It is more important to identify clinical signs of inflammation. Probing depths vary depending on the soft tissue’s height at the implant’s location. However, Rams et al., 1984 demonstrated that relatively healthy implants with stabilized pockets < 5 mm had only 2.3% spirochetes in differential microscopic counts, while implants with deeper pocket formation contained significantly higher levels of anaerobic bacteria.21 Becker 1990 showed that probing depth was greater than 6 mm in 58% of the sites of failing implants.22 Sulcus depths greater than 5 to 6 mm around implants have a greater incidence of anaerobic bacteria, increasing the risk of peri-implant diseases. Probing not only measures pocket depth but also reveals tissue consistency, bleeding and the presence of exudate.
The bleeding on probing and/or suppuration has been indicated as one of the parameters for detecting peri-implant diseases. Bleeding on probing (BOP) has been used as an indicator to evaluate tissue inflammatory response to the presence of infection. According to Lang et al., 1990, the absence of BOP represents reliable indices of periodontal stability. For diagnosing periodontitis, the absence of BOP is highly specific (88%) for periodontal health despite a low sensitivity of 29%. Dukka et al., 2021 evaluated the value of bleeding on probing to assess peri-implant diseases as more intricate.23 Dukka concluded that the presence of BOP around implants also may or may not suggest ongoing inflammation, and the absence of bleeding on probing confirms health.24 The assessment value of using BOP as a diagnostic tool for peri-implant disease may fluctuate between 0% to 52%. BOP should also be used in addition to other parameters such as probing depth, progressive bone loss and visualization of inflammation. Suppuration is another one of the parameters. Monje et al. 2021 showed the association of the presence and grade of suppuration with peri-implant bone loss, probing depth and defect morphology in patients with peri-implantitis.25 However, suppuration in peri-implant mucositis is contentious.24 2017 World Workshop concluded that clinical signs of inflammation, including redness, edema, mucosal enlargement, BOP+ with or without suppuration, along with increases in PD and radiographic bone loss, are commonly used in case definitions for peri-implantitis.26
The osseointegrated implant should be rigidly anchored (ankylosed) to the surrounding bone and present without clinical mobility with vertical or horizontal forces under 500 mg.27,28,29 Any detected implant mobility indicates failure. A healthy implant may move less than 75 µm, which cannot be observed clinically.30 Implant prostheses also should present with no clinical mobility. Implant prosthesis mobility can lead to crestal bone loss and, if not addressed in time, may lead to implant mobility and failure.
Several classifications of peri-implant defect are quoted in the literature, but no uniformly accepted classifications. Success criteria for endosteal implants have been proposed previously by other authors, including Schnitman and Shulman, Cranin et al., McKinney et al., Albrektsson et al., and Albrektsson and Zarb. An implant quality of health scale with five levels has been established by James and modified by Misch.31 The James-Misch scale also proposes management modalities corresponding to five levels: optimum health, satisfactory health, compromised health, clinical failure, and absolute failure.29,31
Misch et al., 2008 proposed four groups of implant quality scale: success (optimum health), satisfactory survival, compromised survival and failure (clinical or absolute failure).32 Each category has clinical conditions utilizing parameters such as pain, tenderness upon function, mobility, radiographic bone loss from the initial surgery, probing depth, and history of exudate. Each category also has proposed management associated with clinical conditions.31,32
Froum & Rosen 2012 proposed the classification of peri-implantitis into early, moderate, and advanced utilizing probing depth and percentage of radiographic bone loss with possible bleeding and/or suppuration on probing.33
The classification of peri-implant diseases and implant quality scale allows the implant dentist to evaluate an implant using various parameters and manage the implant accordingly. The current classification (Suzuki – Hsiao – Resnik) highlights implant health and disease categories and includes treatment strategies for each group.
Table 1: Implant Quality Health Scale
| Implant Quality Scale | Clinical & Radiographic Conditions | Management |
| Implant Health Osseointegration No Bone Lossa | No pain or tenderness upon function 0 mobility < 2 mm radiographic bone loss after complete bone remodelinga PD ≤ 4 mmb (no bleeding and/or suppuration on probing) No implant thread exposure Acceptable occlusion guideline (reference chapter) | Normal maintenance Patient self-administered care Occlusal adjustment prn |
| Peri-implant Mucositis Acceptable Health No Bone Lossa | No pain or tenderness 0 mobility < 2 mm radiographic bone loss after complete bone remodelinga Peri-mucosal inflammationPD ≥ 4 mmb (Bleeding and/or suppuration on probing) No implant thread exposure Peri-mucosal inflammation Presence of etiologies: food debris, plaque, cement, etc. May have inadequate gingiva support, compromised occlusion/prosthetic design | Frequent periodontal and/peri-implant maintenance Nonsurgical debridement (hand, machine, air power, lasers etc) Patient self-administered care Adjunct local and systemic antimicrobial Soft tissue and/or prosthetic corrections if required. Occlusal adjustment prn |
| Early Peri-implantitis Potentially Compromised Health | No pain or tenderness 0 mobility | Lasers (LAPIP, NdYAG) |
| Mild bone Loss | 2-4 mm radiographic bone loss after complete bone remodeling PD ≥ 4 mmb (bleeding and/or suppuration on probing) Approximately 1-2 implant thread exposurec Peri-mucosal inflammation Bone loss < 25% of the implant length Presence of etiologies: food debris, plaque, cement, etc. May have inadequate gingiva support, compromised occlusion/prosthetic design | |
| Moderate Peri-implantitis Compromised Health Moderate Bone Loss | Variable pain or tenderness 0 mobility PD ≥ 6 mm2 (bleeding and/or suppuration on probing) Approximately 3-5 implant thread exposurec Peri-mucosal inflammation Bone loss 25-50% of the implant length Presence of etiologies: food debris, plaque, cement, etc. May have inadequate gingiva support, compromised occlusion/prosthetic design | Treatment as early peri-implantitis plus surgical re-entry (resective or regeneration) Lasers (LAPIP, NdYAG) Implant surface decontamination |
| Advanced Peri-implantitis Very compromised Health Advanced Bone Loss | Variable pain or tenderness ± mobility PD > 8 mm2 (bleeding and/or suppuration on probing) 5 implant thread exposurec Peri-mucosal inflammation Bone loss > 50% of the implant length | Treatment as early-peri-implantitis plus surgical re-entry (resective or regeneration) Lasers (LAPIP, NdYAG) Implant surface decontamination Implantoplasty |
| Prognosis: Unfavorable to Hopeless | Presence of etiologies: food debris, plaque, cement, etc. May have compromised occlusion/prosthetic design | Removal of implant |
| Retrograde peri-implantitis | Variable peri-mucosal inflammation Radiographically: periapical lesion around implant Clinical: pain, tenderness, fistula formation or swelling | Address etiologies Surgical re-entry Implantoplasty or removal of implant |
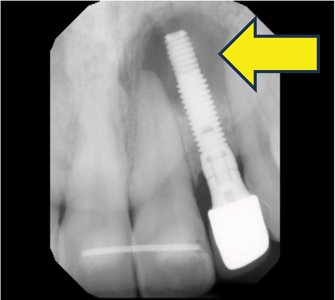
Implant Health (Figs. 1A,B)
For implant health, the implant is osseointegrated with no bone loss. It should succeed with no pain or tenderness observed with palpation, percussion, or function, and no implant mobility or bone loss is detected after biologic and functional remodelling after delivery of implant prosthesis.
With biologic and functional remodelling, there should be less than 2 mm crestal bone loss radiographically since the implant placement. The probing depth is less or equal to 4 mm and is stable after the first year if the implant is placed according to following the cemental-enamel junction (CEJ) of adjacent teeth. However, probing depths may vary depending on implant submergence depth, implant platform, implant-abutment connection, prosthetic design, etc. The implant has no history of bleeding and exudate, and no radiolucency is present around the implant body. There is no implant thread exposure clinically with acceptable occlusion guidance that does not lead to heavy occlusion and implant marginal bone loss. In standard management for implants presented with health, it is essential to ensure the patient can perform adequate self-administered home care. Occlusion should be monitored during maintenance visits and adjusted accordingly from functional wear.
Fig. 1A

Fig. 1B

Peri-implant mucositis (Figs 2A & 2B)
For peri-mucositis, implants exhibit acceptable health. There is no bone loss as with implant health with less than 2 mm radiographic bone loss after complete bone remodelling. There is no observable implant mobility. No pain or tenderness is observed with palpation, percussion, or function. Peri-mucosal inflammation is present with bleeding and/or suppuration on probing.
The probing depth is less or equal to 4 mm, and no implant threads are exposed. Implants with peri-implant mucositis present with aetiologies, such as plaque and food debris, that lead to peri-mucosal inflammation. The implant site may present with inadequate soft tissue for support.
Implant occlusion and prosthetic design may be compromised, leading to soft tissue inflammation. Management for peri-implant mucositis consists of frequent periodontal and/or peri-implant maintenance with the presence of aetiologies and, depending on the depth, non-surgical debridement with hand instruments, ultrasonics, piezo, air powder, lasers, etc. Depending on the systemic and local conditions, Adjunct local and systemic antimicrobials may be required. Soft tissue and/or prosthetic corrections and occlusal adjustments may be necessary for return to implant health.
Fig. 2A

Fig. 2B

Early Peri-implantitis (Figs. 3A & 3B)
For early peri-implantitis, implants exhibit potentially compromised health. Mild bone loss occurs with 2 to 4 mm radiographic bone loss after complete bone remodelling. There is no observable implant mobility. No pain or tenderness is observed with palpation, percussion, or function.
Peri-implant mucosal inflammation is present with bleeding and/or suppuration on probing. The probing depth is less or equal to 4 mm. Approximately 1 to 2 implant threads are exposed. However, implant thread loss may vary depending on implant design and length.
Bone loss is approximately < 25% of the implant length. Implants present with aetiologies, such as plaque and food debris, that lead to early bone loss. Implant sites may present with inadequate soft tissue or thin biotype (phenotype). Implant occlusion and prosthetic design may be compromised, leading to soft and hard tissue deficiency.
Management for early peri-implantitis is similar to peri-implant mucositis, which consists of periodontal and/or peri-implant maintenance, non-surgical therapy, adjunct local and systemic antimicrobial therapies, soft tissue and/or prosthetic corrections and occlusal adjustments.
Fig. 3A & 3B
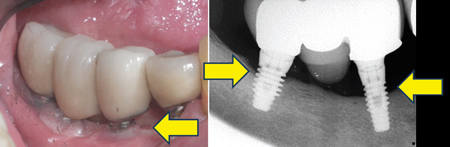
Moderate Peri-implantitis (Figs. 4A,B,C,D)
For moderate peri-implantitis, implants exhibit compromised health. Moderate bone loss with no observable implant mobility and pain or tenderness may be present with palpation, percussion, or function. Peri-implant mucosal inflammation is present with bleeding and/or suppuration on probing. The probing depth is less than and equal to 6 mm. Approximately 3 to 5 implant threads are exposed.
However, the number of implant threads involved may vary depending on implant design and length. Bone loss is approximately 25 to 50% of the implant length. The implant presented with aetiologies such as plaque, food debris, and cement that lead to moderate bone loss. Implant sites may present with inadequate soft tissue for support. Implant occlusion and prosthetic design may be compromised, resulting in soft and hard tissue deficiency.
Management for moderate peri-implantitis includes treatments such as peri-implant mucositis and early peri-implantitis. It consists of periodontal and peri-implant maintenance, non-surgical therapy, adjunct local and systemic antimicrobial therapies, soft tissue and/or prosthetic corrections and occlusal adjustments.
However, non-surgical therapy is insufficient to access and address the aetiologies adequately. Surgical re-entry of resection or regeneration is required to access the implant surface for decontamination.
Fig. 4A

Fig. 4B

Fig. 4C
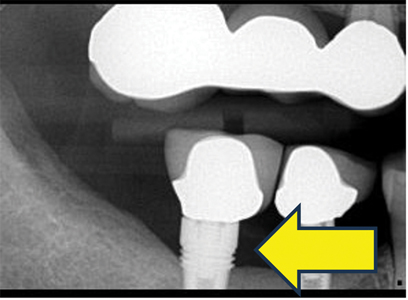
Fig. 4D
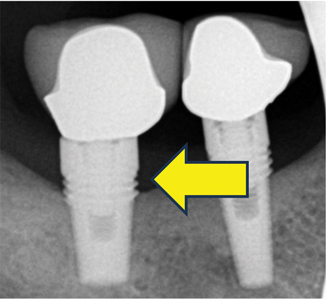
Advanced Peri-implantitis (Figs. 5A, & 5B)
For advanced peri-implantitis, implants exhibit significantly compromised health. Advanced bone loss is present. Implants may or may not have mobility. Pain or tenderness may be present with palpation, percussion, or function. Peri-implant mucosal inflammation is present with bleeding and/or suppuration on probing.
The probing depth is more than and equal to 8 mm, and more than five implant threads are exposed. However, the number of implant threads lost may vary depending on implant design and length. Bone loss is more than 50% of the implant length.
Implants present with aetiologies such as plaque, food debris, and cement that lead to advanced bone loss. The implant site may present with inadequate soft tissue for support. Implant occlusion and prosthetic design may be compromised, leading to soft and hard tissue deficiencies. Management for advanced peri-implantitis depends on whether the implant may be rescued and maintained.
Non-surgical treatments remove aetiology and reduce inflammation and infection. Like the moderate peri-implantitis group, non-surgical therapy cannot adequately access and address the aetiologies. Surgical re-entry of resection or regeneration is required to access the implant surface for contamination. If the implant is mobile or cannot be adequately decontaminated and maintained, the implant will be removed surgically.
Fig. 5A
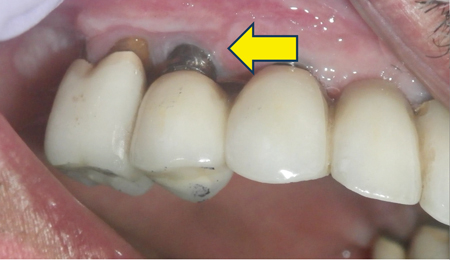
Fig. 5B
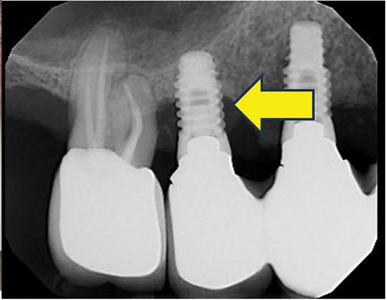
Retrograde Peri-Implantitis (Fig. 6)
For other peri-implant diseases such as retrograde peri-implantitis (periapical peri-implantitis), periapical radiolucency lesion is observed radiographically. Clinical signs may include pain, tenderness, formation of fistula, and swelling. There may be variable peri-implant mucosal inflammation.
The clinical and radiographic signs of inflammation are noted between 2 to 8 weeks and up to 4 years after implant placement, with most of the reports associated with endodontic lesions of adjacent teeth.26
Management for retrograde peri-implantitis may address etiologies if an adjacent tooth is involved, or root canal therapy may be required. Area of radiolucency and infection may require additional surgical re-entry, implantoplasty, or implant removal.26,34
Identifying implant health and disease conditions is vital for implant prognosis and management. No single diagnostic tool can establish a diagnosis of peri-implant diseases. Various clinical and radiographic assessments of pain, mobility, changes in probing depths, bleeding and/ or suppuration upon probing, implant thread exposure, mucosal inflammation, radiographic bone loss, presence of aetiologies, soft tissue support, prosthetic design and occlusal load can assist in determining the implant quality.
Fig. 6
Conclusion
The current implant health scale (Suzuki – Hsiao – Resnik) provides a framework for assessing implant health. It also provides specific criteria for diagnosing stages of peri-implant diseases. Suggested treatment modalities for each stage of implant health and disease are included for the guidance of implant clinicians.
However, treatment approaches may differ and be modified according to a patient’s overall systemic health, level of plaque biofilm, history of periodontal disease, occlusal factors, prosthetic design, and habits such as smoking, among other clinical factors. Proper implant maintenance visits and plaque control are the most consistent factors supporting optimal long-term implant health.
Oral Health welcomes this original article.
References
- Branemark R, Brenemark PI, Rydevik B, Myers RR. Osseointegration in skeletal reconstruction and rehabilitation: a Review. J. Rehabil Res Dev. 2001 Mar-Apr;38(2):175-81.
- Carisson L, Rostlund T, Albrektsson B, Albrektsson T, Branemark PI. Osseointegration of titanium implants. Acta Orthop Scand. 1986 Aug;57(4);285-9.
- Flanagan D. Osseous Remodeling around dental implants. J Oral Implantol. 2019 Jun;45(3):239-246.
- Kyzyk P, Schemitsch EH. The basic science of peri-implant bone healing. Indian J. Orthop. 2011 Mar;45(2):108-14.
- Bouri A. Jr, Bissada N., Al-Zahrani MS, Faddoul F, Nouneh I. Width of keratinized gingiva and the health status of the supporting tissues around dental implants. Int’l J. Oral Maxillofac Implants. 2008 Mar-Apr;23(2):323-6.
- Kim BS, Kim YK, Yun PY, Yi YJ, Lee HJ, Kim SG, Son JS. Evaluation of peri-implant tissue response according to the presence of keratinized mucosa. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009 Mar;107(3):e24-8.
- Lin GH, Chan HL, Wang HL. The significance of keratinized mucosa on implant health: a systematic review. J Periodontol. 2013 Dec;84(12):1755-67.
- American Academy of Periodontology 2017 World Workshop Consensus. J.Perio-dontol. 2018. Chicago, IL. USA
- American Academy of Periodontology. Peri-implant mucositis and peri-implantitis: a current understanding of their diagnoses and clinical implications. J. Periodontol. 2013 Apr; 84(4):436-43.
- Adell R, Lekholm U, Rockler B, Brånemark PI. A 15-year study of osseointegrated implants in the treatment of the edentulous jaw. Int J. Oral Surg. 1981 Dec;10(6):387-416.
- Lindquist LW, Carlsson GE, Jemt T. A prospective 15-year follow-up study of mandibular fixed prostheses supported by osseointegrated implants. Clinical results and marginal bone loss. Clin Oral Implants Res. 1996 Dec; 7(4): 329–36.
- Esposito M, Hirsch JM, Lekholm U, Thomsen P. Biological factors contributing to failures of osseointegrated oral implants. (I) Success criteria and epidemiology. Eur J Oral Sci 1998 Feb; 106(1): 527–51.
- De Smet E, Jacobs R, Gijbels F, Naert I. The accuracy and reliability of radiographic methods for the assessment of marginal bone level around oral implants. Dentomaxillofac Radiol. 2002 May;31(3): 176–81.
- Romeo E, Lops D, Margutti E, Ghisolfi M, Chiapasco M, Vogel G. Long-term survival and success of oral implants in the treatment of full and partial arches: a 7-year prospective study with the ITI dental implant system. Int J Oral Maxillofac Implants. 2004 Mar-Apr;19(2):247–59.
- Albrektsson T, Isidor F. Consensus report of session IV. In: Lang NP, Karring T, eds. Proceedings of the 1st European Workshop on Periodontol. London, UK: Quintessence Publishing Co, Ltd; 1994:365–9.
- Albrektsson T, Zarb G, Worthington P, Eriksson AR. The long-term efficacy of currently used dental implants: A review and proposed criteria of success. Int J Oral Maxillofac Implants. 1986 Summer;1(1):11–25.
- Geraets W, Zhang L, Liu Y, & Wismeijer D. Annual bone loss and success rates of dental implants based on radiographic measurements. Dentomaxillofac Radiol. 2014;43(7):20140007.
- Laurell L, Lundgren D. Marginal bone level changes at dental implants after 5 years in function: a meta-analysis. Clin Implant Dent Relat Res. 2011 Mar;13(1):19-28.
- Greenstein G, Cavallaro J, Tarnow D. Dental Implantology: Numbers Clinicians Need to Know. Compend Contin Educ Dent.2019 May;40(5):e1-e26.
- Berglundh T, Armitage G, Araujo MG, et al. Peri-implant diseases and conditions: consensus report of workgroup 4 of the 2017 World Workshop on the classification of periodontal and peri-implant diseases and conditions. J Periodontol. 2018 Jun;89 Suppl 1:S313-S318.
- Rams TE, Roberts TW, Tatum H Jr, Keyes PH. The subgingival microbial flora associated with human dental implants. J Prosth Dent 1984 Apr;51(4):529-534.
- Becker W, Becker BE, Newman MG, et al. Clnical microbiologic findings that may contribute to dental implant failure. Int J Oral Maxillofac Implants. 1990 Spring; 5(1):31-8.
- Lang NP, Adler R, Joss A, Nyman S. Absence of bleeding on probing. An indicator of periodontal stability. J Clin Periodontol. 1990 Nov;17(10):714-721.
- Dukka H, A Saleh MH, Ravida A, et al. Is bleeding on probing a reliable clinical indicator of peri-implant diseases? J Periodontol. 2021 Dec;92(12):1669-1674.
- Monje A, Vera M, Muoz-Sanz A, Wang HL, Nart J. Suppuration as diagnostic criterion of peri-implantitis. J Periodontol. 2021 Feb; 92(2):216-224.
- Schwarz F, Derks J, Monje A, Wang HL. Peri-implantitis. J Clin Periodontol. 2018 Jun;45 Suppl 20:S246-S266.
- Araujo MG & Lindhe J. Peri-implant health. J Clin Periodontol. 2018 Jun;45 Suppl 20:S230-S236.
- Misch CE, Perel ML, Wang HL, et al. Implant success, survival, and failure: The International Congress of Oral Implantologists (ICOI) Pisa Consensus Conference. Implant Dent. 2008 Mar;17(1):5–15.
- Misch CE. Implant quality scale: a clinical assessment of the health disease continuum. Oral Health. 1998 Jul;88(7):15-20, 23-5.
- Sekine H, Komiyama Y, Hotta H, et al. Mobility characteristics and tactile sensitivity of osseointegrated fixture-supporting systems. In: van Steenberghe D, ed. Tissue Integration in Oral Maxillofacial Reconstruction. Amsterdam: Excerpta Medica; 1986:326-329.
- Resnik R. Ed. Misch’s Avoiding Complications in Oral Implantology 1st edition, Elsevier-Mosby, St. Louis, USA 2017. P. 2066.
- Misch CE. Contemporary implant dentistry 3rd edition. Mosby Elsevier, St. Louis, USA 2008. Chapter 42.
- Froum SJ, Rosen PS. A proposed classification for peri-implantitis. Int J Periodontics Restorative Dent. 2012 Oct;32(5):533-40.
- Quirynen M, Vogels R, Alsaadi G, Naert I, Jacobs R, van Steenberghe D. Predisposing conditions for retrograde peri‐implantitis, and treatment suggestions. Clin Oral Implants Res. 2005;16:599–608
About the Authors

Dr. Jon B. Suzuki, Clinical Professor, University of Maryland, Baltimore, MD, USA. Clinical Professor, University of Washington, Seattle, WA, USA. Clinical Professor, Nova-Southeastern University, Ft. Lauderdale, FL USA. Professor Emeritus, Temple University, Philadelphia, PA, USA.

Dr. Yueh J. Hsiao, Program Director (Graduate Periodontology and Oral Implantology), Associate Professor, Temple University, Philadelphia, PA, USA.

Dr. Randy R. Resnik, Clinical Professor, Temple University, Philadelphia, PA, USA. Attending, Allegheny General Hospital, Pittsburgh, PA, USA.









