Published reports in the 1960s investigated reported increases in upper respiratory tract infections in dentists from airborne splashes, spatter, and aerosols generated during use of high-speed handpieces. Later studies also indicated a higher incidence of colds in dental hygienists who did not routinely wear masks during patient care. Despite demonstrations of occupational risk from airborne bioburden, many dentists, hygienists, and assistants did not routinely wear face masks during treatment procedures prior to 1980. As infection control awareness increased, prescribed protective measures began to address the heightened interest in respiratory protection for all dental professionals. Beginning with the initial 1978 American Dental Association (ADA) published document, every series of ADA and Centers for Disease Control and Prevention (CDC) infection control guidelines for dentistry has included recommendations for wearing face masks as a key component of personal protection against airborne pathogens. The 1991 Occupational Health and Safety Administration (OSHA) Bloodborne Pathogens Standard also included a regulation stating: “Masks in combination with eye protection devices…. shall be worn whenever splashes, spray, spatter, or droplets of blood or other potentially infectious materials may be generated and eye, nose, or mouth contamination can be reasonably anticipated.”
Multiple routes of cross-contamination and infection are present in healthcare settings; these include:
1) Direct contact with body fluids.
2) Indirect contact with sharps, instruments and other contaminated treatment items.
3) Exposure to airborne infectious particles.
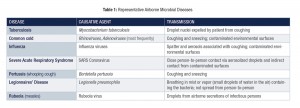
With regard to the latter, there is an extensive history of diseases transmitted by airborne bacteria and viruses (Table 1). As scientific and clinical knowledge increased concerning the infectivity of airborne spatter (particles > 50 µ in diameter) and aerosols (0.5-50 µ particles), the rationale for wearing masks by healthcare professionals (HCP) expanded to include protection of HCP and not only their patients. There can be extensive generation of airborne material during the majority of dental procedures. Spatter, containing large particulate matter and aerosols with visible fluid, is often clearly seen during procedures. These droplets are propelled from intraoral operative sites and remain airborne only briefly because of their large size. However, aerosols are able to stay airborne for much longer intervals and have the potential to enter unprotected bronchioles and alveoli of the lungs. Ninety-five percent of dental aerosols are 5.0 µ or less in diameter and cannot be seen. Microorganisms isolated from these particles include staphylococci, pneumococci, tubercle bacilli, influenza viruses, hepatitis viruses, rhinoviruses, and herpes viruses.
Mask Performance Standards
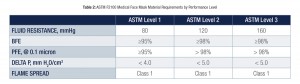
The American Society of Testing and Materials (ASTM) is the organization responsible for establishing criteria and testing methods to delineate performance specifications for face masks used in healthcare. ASTM published an updated description of performance requirements in 2011, and these are recognized as the industry standard for healthcare face masks (Table 2). Masks are generally classified into 3 types. These categories are: low (ASTM Level 1), moderate (ASTM level 2), and high barrier (ASTM Level 3). Approved test information is described on the product box using the terms bacterial filtration efficiency (BFE) and particle filtration efficiency (PFE). BFE is measured using viable particles (e.g. bacteria) ranging in size from 1-5 µ, while PFE is determined using fixed-size, non-viable particles measuring 0.1-1.0 µ.
FLUID RESISTANCE:
> represents mask’s resistance to penetration by synthetic blood under pressure (mmHg)
> measures ability of mask’s construction to minimize fluids from traveling through the material and potentially coming into contact with the wearer
> higher the fluid resistance (filtration), the better the protection
BFE (Bacterial Filtration Efficiency):
> represents percentage of bacteria filtered out at pore size of 1–5 microns
> the measure of efficiency of the mask filtering bacteria through it
PFE (Particulate Filtration Efficiency):
> represents percentage of particles filtered out at a pore size of 0.1–1.0 microns
> the measure of the efficiency of the mask in filtering particles passing through it
> the size of the particles filtered is critical
DELTA P (Differential Pressure):
> represents the pressure drop across the mask or resistance to air flow in mmH2O/cm2
> determines breathing resistance
> higher the Delta P, the less breathability, but the better the filtration
FLAME SPREAD:
> measures flame spread of the mask material
Source: The American Society for Testing and Materials. Standard specification for performance of materials used in medical face masks. F2100-11 Standard.
The BFE and PFE are important criteria used to distinguish the three ASTM mask levels. The higher the documented percentages for a mask, the better protective performance in preventing microbial penetration. ASTM Level 1(low barrier) masks are designed to be used during patient care where low concentrations of spatter, fluid, or aerosols are generated. Examples include intraoral examinations, taking impressions, and orthodontics procedures. A higher level of respiratory protection is afforded by ASTM Level 2 masks. These are manufactured to provide a protective barrier when treatment produces moderate amounts of airborne fluid, spatter and/or aerosols. Examples of mask use here would include most routine restorative procedures and when performing scaling and root planing. The greatest resistance to particle penetration and best provider protection occurs when one uses ASTM Level 3 masks. These single-use disposables are to be worn when heavy to moderate airborne material is produced, such as levels noted during surgical, ultrasonic scaling, and long, extended restorative procedures (e.g. crown preparation).
Proper Use and Misuse
As with any infection control practice, an important factor for achieving maximum mask effectiveness is wearer compliance. Multiple issues can occur in clinical practice, which can reduce the level of mask protection.
First, if a mask does not cover the nostrils of the HCP because it is “too hot” for the wearer, then the mask is obviously not effective. The solution is to find a more comfortable mask so it can be worn properly.
Second, aerosolized material can enter a HCP’s respiratory tract if the mask does not fit well. This can occur when gaps are present on the sides allowing airborne microbes to enter. In this instance, use of a “one size fits all” approach to mask selection may not provide different users with appropriate protection.
The solution is for the HCP to use a mask that conforms to the shape of the person’s face, affording a more effective seal. Other common problems are wearing face masks too long during extended treatment procedures and not changing the mask between patients. Masks have a use-life. It is important to remember that masks are manufactured as single-use disposable items. They become wet on the outside with prolonged wear and exposure to spatter and aerosols. In addition, the wearer’s breath also moistens the inside fabric layer. A mask can lose its protective filtration quality over time as a result of fluid exposure from both sides, and thus become a nidus of microbial accumulation. Airborne microorganisms can subsequently penetrate through the wet fabric by a process called “wicking.” Wet masks should therefore be changed every 20 minutes when challenged during procedures that generate heavy levels of spatter and aerosols, and after 60 minutes in non-aerosol environments. The 2003 CDC dental infection control guidelines address these issues: “Change masks between patients or during patient treatment if the mask becomes wet.”
HCP have a responsibility to protect both their patients and themselves. The design and filtration capabilities of today’s face masks have been shown to protect against microbial challenges encountered during provision of care. One should also understand that no mask can filter out 100 percent of aerosolized particles.
By applying a few basic criteria HCP will be better prepared to make safer, effective choices to minimize occupational airborne infections.OH
Dr. John Molinari is currently Director of Infection Control for THE DENTAL ADVISOR in Ann Arbor, Michigan. Previously, he served for 32 years at the University of Detroit Mercy School of Dentistry as Professor and Chairman of the Department of Biomedical Sciences and Director of Infection Control. He has published over 450 scientific articles, text chapters, and abstracts in the areas of microbiology and immunology, and lectures nationally and internationally on topics dealing with infectious diseases and infection control. He was previously a member of the Michigan Board of Dentistry. In recognition of his efforts, Dr. Molinari was inducted as an honorary member of the Michigan Dental Association, the International College of Dentists, the American College of Dentists, and was a recipient of the ADA Golden Apple Award in 2009.
Ms. Peri Nelson graduated from the University of Michigan in 2003 with a Bachelor of Science in Neuroscience and Biology. Her research experience is in the fields of microbiology, genetics, neuroscience and biomaterials. Peri has educated thousands of dental professionals and students through writing, webinars, and hands-on training. She currently works as a Senior Research Associate in the Infection Control Laboratory with Dr. John Molinari.
Oral Health welcomes this original article.
References:
1. ADA Councils on Dental Therapeutics. Infection control in the dental office. J Amer Dent Assoc. 1978;97:673-677.
2. Department of Labor, Occupational Safety and Health Administration. Occupational exposure to bloodborne pathogens; final rule. Fed Reg 1991; 56:64004-64182.
3. Micik RE, Miller RL, Mazarella MA, et al. Studies on dental aerobiology: I. Bacterial aerosols generated during dental procedures. J Dent Res 1969;48:49-56.
4. CDC. Guidelines for infection control in healthcare settings. Morbid Mortal Wkly Rpt. 2003; 52:1-66.