Disease prevalence in dentistry is an ever-increasing battle. As dental hygienists, being the true prevention specialists, it is difficult not to become frustrated or even deflated by the statistics surrounding periodontal disease and the high caries rates. Our assessment strategies have come a long way. However, perhaps it is time to implement new techniques and more specialized tools to assess personal levels of risk and tailor treatment to specific, individualized needs.
Patients are actively seeking ways to improve both their oral and overall health. A new model of wellness has emerged, focusing on improving overall patient well-being rather than treating a specific disease and its symptoms alone. By combining precision medicine and a comprehensive approach to health, we can incorporate new diagnostics, risk assessments, patient involvement, and technological advances to enhance healthcare delivery. Achieving the goal of transitioning from a disease to health focus requires clear guidelines that characterize the various stages of health and disease (such as gingivitis, periodontitis, and caries disease). It is crucial to understand the levels of intervention necessary to drive these transitions toward health.1
The way a patient will respond to associated risks and treatment is as unique as their fingerprint.2 For example, some patients may manifest an extensive biofilm presence coupled with minimal host response, while others may exhibit minimal biofilm presence with a substantial host response. This is a scenario that many of us can relate to and may have felt frustration around in the past. Various causative factors determine the immune blueprint and, consequently, the host response to various risk factors and treatment recommendation.3 There are two different chairside salivary tests available, genetic and bacterial, that may help determine the risk factors present. One or both tests can enhance diagnostic data and aid in identifying the ideal treatment recommendation for the patient’s unique needs. The first test we will discuss is new to dentistry and assesses the patient’s own genetic predisposition to periodontal disease and caries, while the second assesses the pathogenic burden of the individual by identifying the type and load of bacteria present. This article will highlight the benefits of both and illustrate how they can work synergistically to form a comprehensive risk assessment protocol with the aim of prevention.
Genetic Testing
- Determines if a patient is genetically predisposed to periodontal or caries disease based on their genetic DNA compared against a population baseline derived from identifying genetic variants through Genome-Wide Association studies.4
- Is only required once in a lifetime as genetic predisposition does not change.
- Offers objective data to help formulate personalized care plans.
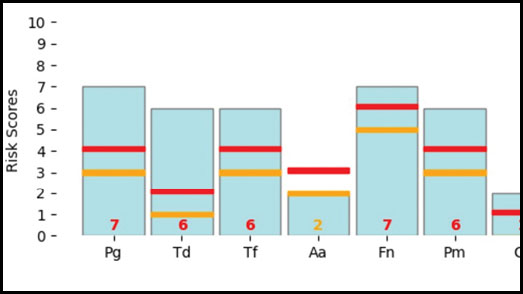
- Provides a report identifying patient risk for periodontal disease or caries as low, moderate or high (Fig. 1).
- Offers early detection of risk to enable proactive care even prior to clinical manifestations.
- Improves patient engagement and compliance.
- Enables action to be taken to prevent and change potential negative outcomes.
Fig. 1
Bacterial Testing
- Serves as a motivating tool for patients, enabling them to actively track and monitor the load and type of bacteria present.
- Integration of testing into protocols facilitates collaboration and allows for a more comprehensive approach to addressing potential health concerns.
- Provides a more informed and tailored approach to healthcare.
- Testing can effectively confirm the presence of symbiotic or dysbiotic biofilm.
- Plays a crucial role in identifying potential concerns such as periodontal disease, caries disease, pathogenic bacteria or yeast overload.
- Can be added to help monitor implant health.
- Can be used to evaluate the effect of certain products or treatments on bacterial load.
- Assists in establishing the important link between oral health and overall body wellness for the patient.
Incorporating testing into the dental appointment
Utilizing testing is an opportunity to understand risk factors for the patient before they present with active disease, bone loss, or a failing implant. It can be collected by dental assistants (depending on their scope of practice), dental hygienists, dentists and/or denturists. The most common time to perform one or both tests is during an initial hygiene appointment or at recare. Testing is recommended as a risk assessment tool not a diagnosis. These results help to uncover patient risk and then to formulate the ideal treatment plan for the patient.
To identify individuals who can benefit from a test, it is important to carefully review the patient’s chart and then determine the type of test to recommend. A genetic test may be indicated if the patient has a familial, clinical, or medical history that includes high risks for periodontal disease, caries, diabetes, smoking, obesity, xerostomia, stress, and/or dietary concerns. It may also be indicated prior to orthodontic treatment, veneers, crown bridge and implants, to determine the patient risk profile for success. In periodontal disease and caries, as in many other infections, the manifestation of the diseases depends on the host’s immune response, which is highly influenced by genetics.5 A genetic test can unlock some of the answers behind certain manifestations and/or responses.
It may be necessary to conduct a bacterial test for the patient based on their genetic testing results, current dental status (including gingivitis, periodontal concerns, and caries), as well as complaints of bad taste, halitosis, or tonsil stones. Additionally, it is important to review radiographs for any changes or concerns related to implants. Furthermore, delving into the patient’s health history to identify risk factors such as smoking, diabetes, and cardiovascular disease is essential in determining the need for a bacterial test.
Case Scenarios
Creating the most effective and personalized treatment plan requires careful consideration of various risk factors (Fig. 2) such as the patient’s medical history, current symptoms, and individual preferences. It is a complex process that involves close collaboration between healthcare providers and patients. Identifying the need for conducting the test is the first step.
Fig. 2: Risk Factors
- Radiographic changes
- Genetic predisposition
- Family history of oral disease
- Comorbidities
- Obesity
- Lifestyle factors
- Poor oral hygiene
- Clincal attachment loss and BOP
- Biofilm amount (Disclosing )
- Halitosis, bad taste, tonsil stones
- Moderate to severe gingivitis
- Stage 1 – 4 periodontitis
- Remission or stability
- Implant disease
- Caries dieases
Genetic Testing
Genetic Testing can have a significant impact on a patient’s level of understanding, motivation, and collaboration with their oral health providers. The way the results impact the patient will be very case specific, but let’s consider one such case together.
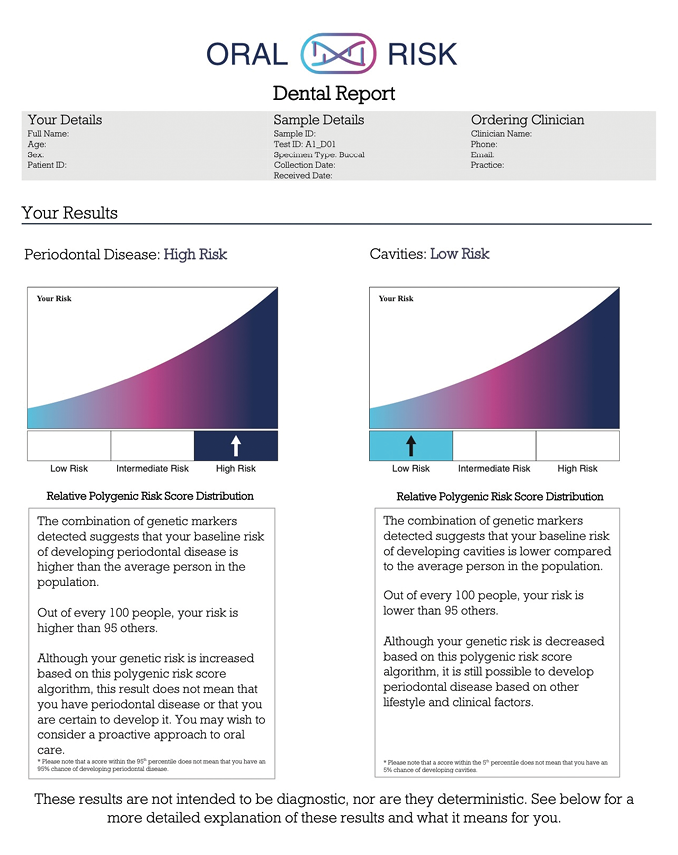
A 50-year-old female patient made the decision to obtain veneers from her 1st quadrant first premolar to her 2nd quadrant first premolar. To be able to make an informed decision and to understand her risk profile, a genetic test was taken. The results for caries disease came back as low-risk, but the report for periodontal disease placed her on the high-risk side (Fig. 3). As this elective treatment was very important to her, the test helped her feel empowered to make the investment while understanding her risk profile. She decided to proceed but wanted to know how she could reduce her risk for periodontal disease and how she could protect her smile, health, and her investment. The patient opted to increase her maintenance visits to every 3-4 months and reported becoming much more engaged in the periodontal charting and assessment phase of her treatment than she had been previously. For prevention, regular radiographs, and the use of the PerioProtect Perio TrayTM system were implemented as well as interproximal brushes and a power toothbrush. The patient has become very diligent with her oral health because of understanding her genetic risk profile and the power she had to act and influence the outcome.
Fig. 3
Bacterial Testing
Depending on the presence of periodontal, caries, genetic risk and/or yeast bacteria risk, our treatment plans need to be customized to meet their specific needs. These may include the use of antibiotic rinses or pills, laser therapy (LBR, LAPT), probiotics, additional biofilm control devices, additional hygiene therapy appointments, or other complementary therapies.
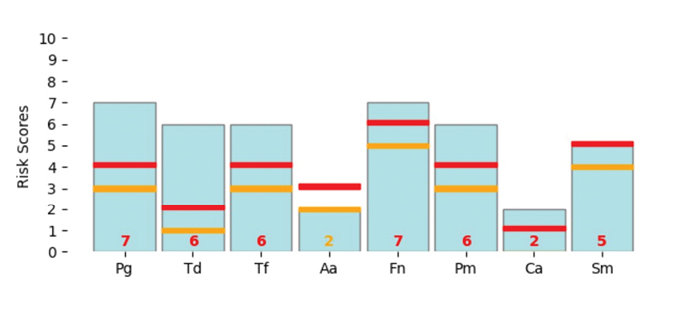
The following patient was specifically selected for a bacterial test because they have Stage 3 Grade C periodontitis, with peri-implant mucositis present on teeth 14 and 15. Their last hygiene therapy appointment was 3 years ago. Upon reviewing their health history, it was found that they have high blood pressure and Type 2 diabetes with an HbA1c of 7.5. The updated periodontal charting revealed the presence of 5-6mm pockets and 60% Bleeding on Probing (BOP), and the radiographs clearly exhibited bone loss changes exceeding 2mm since the last radiographs were taken. Fig. 4 is the patient’s initial OraVital BiofilmDNA report.
Fig. 4
Based on the patient’s bacterial report risk and their periodontal and medical history, a comprehensive treatment plan was devised, including the following interventions:
- ½ mouth scaling
- Application of fluoride varnish
- Oral hygiene instruction (OHI) with disclosing agent
- Interproximal (IP) cleaning using TePe interproximal brushes
- Utilization of a rubber tip stimulator
- Use of an Electric toothbrush (Oral-B IO)
- OraVital FM4 + Amox antibiotic rinse – used 3 times daily for 2 weeks to reduce the pathogen load
- Laser bacterial reduction during each hygiene appointment to help delay recolonization
- Administration of BioGaia Prodentis Probiotics lozenges 2 times daily for 3 months to support oral health and microbial balance
- Rinsing with OraVital CDLx twice daily to keep the biofilm balanced
- Followed by 3-month hygiene appointments
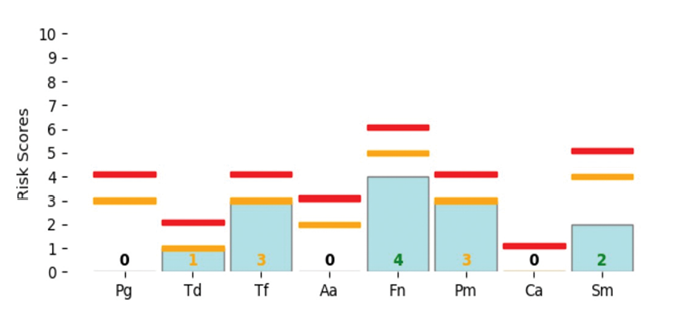
In the patient’s follow-up OraVital BiofilmDNA report 6 months later (Fig.5), we can see the shift in bacterial load and amount. As well we saw a significant reduction in BOP and changes in pocket depths. The patient is extremely motivated to maintain their results.
Fig. 5
Genetic testing and bacterial analysis as part of treatment planning can help ensure a thorough understanding of the risk for and severity of periodontal and/or caries disease. Each province or state has codes within the fee guides that can be used to represent the time spent taking and reviewing the reports with patients. Below is an outline of steps to take to add testing to periodontal protocols.
Steps for the hygiene department to implement testing
- Assess patient risks
- Discuss the Oral Systemic connection
- Review Oral Hygiene Instruction
- Discuss the need for testing, either Genetic, Bacterial, or both – linking it to the medical or family history, periodontal status, biofilm load, dental conditions etc.
- Take the appropriate test(s)
- Complete hygiene therapy – debride, laser, polish, fluoride, etc.
- Get test results back, and follow through on treatment – antibiotic rinses, pills, probiotics, over the counter rinses
- Book patient back for re-evaluation
Conclusion
The current standard for nonsurgical periodontal therapy incorporates periodontal charting and radiographs, followed by various methods of debridement which are often supplemented with antimicrobial agents. Genetic testing can bring us a step ahead in assessing risk rather than waiting for visible changes. Additionally, we can identify and monitor specific pathogenic bacteria exacerbating the host response by implementing bacterial testing. Let’s refrain from speculative assessments of our patients’ risk. It is time to prioritize testing and adopt a proactive strategy for managing periodontal and caries protocols. 
Oral Health welcomes this original article.
References
- Bartold PM, Ivanovski S. P4 Medicine as a model for precision periodontal care. Clin Oral Investig. 2022 Sep;26(9):5517-5533. doi: 10.1007/s00784-022-04469-y. Epub 2022 Mar 28. PMIDAS: 35344104; PMCID: PMC9474478.
- Sivakumar A, Thangaswamy V, Ravi V. Treatment planning in conservative dentistry. J Pharm Bioallied Sci. 2012 Aug;4(Suppl 2):S406-9. doi: 10.4103/0975-7406.100305. PMID: 23066299; PMCID: PMC3467905.
- Loos BG, Van Dyke TE. The role of inflammation and genetics in periodontal disease. Periodontol 2000. 2020 Jun;83(1):26-39. doi: 10.1111/prd.12297. PMID: 32385877; PMCID: PMC7319430.
- StevenD.P.Moore,SolonGuzman,PriscilliaQuan,AprilKennedy,MathewGene, Joel Goodman. Study of a Relative Polygenic Risk Score Assay for Common Oral Health Conditions, Dental Journal Manuscript, November 2022
- Janket SJ, Javaheri H, Ackerson LK, Ayilavarapu S, Meurman JH. Oral Infections, Metabolic Inflammation, Genetics, and Cardiometabolic Diseases. J Dent Res. 2015;94(9 Suppl):119S-27S. doi:10.1177/0022034515580795
About the Authors

Kerry Lepicek, RDH with over 20 years of clinical experience, works at Clinical Research Dental as a Clinical Specialist in Dental Hygiene and Education. Kerry is the editor for The Hygiene Corner with Women in Dentistry, on the Advisory Board for AAOSH, and a cast member on The RDH View. Recently, she won the Ontario Dental Hygienists Association (ODHA) Distinguished Service Award for outstanding contributions to the dental hygiene profession.

Beth Parkes, RDH, BSc with nearly 20 years of clinical experience, is the Manager of Clinical Affairs for Curion, a writer, a board member for Dental Hygiene Quarterly and a cast member of The RDH View. Her overarching goal is to cultivate future leaders within the dental hygiene profession, while helping their patients obtain and maintain optimal oral health.