Introduction
Oral squamous cell carcinoma (OSCC) is a potentially debilitating and deadly disease, particularly when diagnosed at an advanced stage.1 Early detection drastically improves prognostic outcomes, making diagnosis a race against time.2 Most cases of OSCC begin as a precursor lesion or condition, classified by the World Health Organization (WHO) as an “oral potentially malignant disorder (OPMD).”1 The WHO utilizes OPMD as a blanket term for all clinical oral presentations that carry a risk of cancer development (Table 1)1 – though not all disorders will inevitably transform to malignancy.3,4 Lesions commonly progress soon after initial diagnosis, with risk of transformation greatest during the first five years.5 A more detailed account of OPMD characterization can be found in Darling’s initial review of the psoriasin biomarker, published in the December 2018 edition of Oral Health.6
Table 1

The potential risk associated with OPMD cancerous transformation constitutes a significant diagnostic challenge for dentists, often delaying preventive action.3 Clinicians must face the uncertain choice of i) monitoring or ii) surgically removing the lesion; with both options leading to potential negative patient outcomes.1 Known factors, like clinical appearance, can aid with the differential diagnosis of cancer risk, but are not always obvious and/or present.3 The multifactorial complexity of lesion progression, and the absence of distinct clinical indicators, correspond with a limited capacity for the early detection of high-risk lesions.
White lesions of unknown disease or disorder, referred to as leukoplakia, represent the most prevalent type of OPMD.1,4 These lesions are dynamic, and may vary over time in texture and/or colour.3 Clinically, oral leukoplakia can be subcategorized as homogeneous or nonhomogeneous. Homogeneous lesions appear uniformly white, or plaque-like, with a flat or wrinkled surface.3,4 (Fig. 1A) Nonhomogeneous cases may display a warty, nodular or verrucous surface (nodular/verrucous leukoplakia; Fig. 1B), or contain speckled regions of white and red patches (erythroleukoplakia; Fig. 1C).3,4 Red lesions, known as erythroplakia (Fig. 1D), are less common, but present as the highest malignant transforming OPMD.1,3,4 Other patient factors, specifically gender, tobacco use and lesion location, can also influence oral cancer risk.3
Fig. 1A, 1B, 1C, 1D

Lesion Histopathology and Dysplasia Grading
Standard assessment of OPMDs involves acquisition of a tissue biopsy for the histopathological evaluation of preneoplastic changes, referred to as oral epithelial dysplasia (OED).1 Lesion dysplasia is considered the most important prognostic factor of OPMD cancerous transformation. Wide use of the WHO three-tiered grading system stratifies OED into stages of mild, moderate and severe (Fig. 2); with an increase in grade (mild to severe) associated with a higher rate of malignant transformation.1
Fig. 2

This historical categorization of dysplasia grade is arbitrarily defined by the number of atypical oral epithelium thirds; generating a potential for significant i) overlap between grades,1,7 and ii) variability in intraobserver and interobserver scoring.8,9 Variations in pathologist interpretation of dysplasia presence, degree and significance have resulted in a reported examiner agreement ranking of poor-to-moderate.9,10 Consequently, many dysplasia cases do not align with the traditional correlation of dysplasia grade to malignant risk. Severe dysplastic regions may remain static or even regress, while mild dysplastic (or non-dysplastic) lesions have demonstrated malignant capability.11 The validity of dysplasia grading as an independent prognostic tool of oral cancer risk has therefore been challenged – as the presented limitations make it challenging for clinicians to confidently utilize dysplasia grading as a means to guide treatment.
The WHO has recommended application of an updated two-tiered binary dysplasia system to reduce user subjectivity.1,12,13 This revised system utilizes a numerical threshold of abnormal epithelial features (architectural/cytological) to differentiate between low and high-risk lesions – reclassifying cases of no, questionable, or mild dysplasia as low-risk, and cases of severe dysplasia as high-risk. Moderate dysplasias may be recategorized as low- or high-risk lesions.12,13 Limiting dysplasia classification to two categories could improve examiner correlation, amplifying reliability of pathologist diagnosis.
Clear guidelines for the monitoring and/or treatment of dysplastic OPMDs do not currently exist, obstructing patient care. Less than 5% of oral precursor lesions transform to OSCC annually.5 This, coupled with the perceived unpredictability of dysplasia risk assessment, often favours a conservative management of OPMDs.6 Oral cancer diagnoses and/or appropriate treatment accordingly occur at more advanced stages of malignancy, contributing to the high mortality rate of oral carcinomas.1 Elevated trust in OPMD cancerous risk assessment may motivate increased clinician use of preventive procedures, altering time frames for earlier intervention. This depends on the discovery of prognostic biomarkers, and development of a histologic and/or chairside test (using one or multiple biomarkers), to objectively predict the malignant transformation risk of oral lesions.
Biomarkers
Numerous biomarkers have been recognized as prospective indicators of OPMD cancerous transformation, including but not limited to: i) hypermethylation of tumor suppressor genes, ii) loss of genetic heterozygosity, iii) alterations in DNA content and iv) overexpression of psoriasin (S100A7).3,14,15 In recent years, psoriasin has received increasing attention for its proposed correlation to poor prognosis in OSCC patients;15 emerging as the favoured predictive biomarker for oral pre-cancer progression.
Psoriasin is a Ca2+-binding protein of the multigenic Ca2+-modulated S100 family, involved in the regulation of several cellular processes. In normal epithelium, psoriasin expression is most concentrated in the upper, well-differentiated spinous layer – suggesting a more pronounced role in cellular differentiation.16 Overexpression of psoriasin has been observed in several epithelial malignancies, including breast, lung, head and neck, gastric, bladder, cervical and ovarian cancers.17
Psoriasin expression has been experimentally evaluated in both cases of OSCC and OED.15,18 The correlation of total psoriasin presence in oral tumors, to specific clinicopathologic factors, has revealed a stable upregulation of its mRNA in small, well-differentiated, non-metastatic, early stage carcinomas; associating an increase in psoriasin expression with preliminary neoplastic changes.18 Amplified amounts of psoriasin have also been detected in i) a majority of malignant-transforming OED cases, and ii) oral squamous epithelial hyperplasia (prior to any evidence of dysplasia).15 This preneoplastic display of psoriasin demonstrates its enhanced prognostic ability to earlier discern vulnerable patients at greatest risk of malignant progression, through detection of molecular alterations that often precede morphologic changes. Ongoing research continues to analyze psoriasin expression profiles in other OPMDs, including chronic hyperplastic candidiasis.
The precise functional role(s) of psoriasin in OSCC malignancy has yet to be confirmed. A proposed protective role suggests that upregulation of psoriasin corresponds with increased expression of the epithelial adhesion molecule, E-cadherin. Cancer cells traditionally decrease E-cadherin expression to enable tumor migration and infiltration of distant tissue boundaries.19 It has therefore been theorized that the overexpression of psoriasin observed in premalignant lesions serves to stabilize E-cadherin expression, inhibiting cancer invasion.6 Potential carcinogenic functions of psoriasin in tumor differentiation and/or growth have also been described – possibly affiliated with more advanced stages of oral malignancy.6
The Straticyte™ Prognostic Test
Straticyte™ is a novel biomarker application established by Proteocyte AI (Toronto, Canada), that has demonstrated encouraging potential to deliver a personalized five-year risk assessment for the cancerous transformation of an OPMD. Quantitative measurement of the psoriasin biomarker (Fig. 3) is combined with computational algorithms to develop a lesion risk score; established through comparison to a reference database with more than 150 annotated oral dysplasia cases.7
Fig. 3
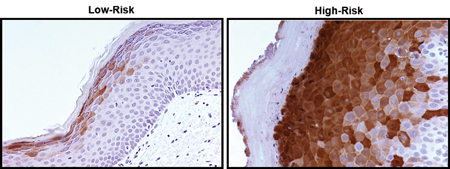
Biopsy samples of OED are classified into three Straticyte™ grades (low-, medium- or high-risk) that exhibit minimal prognostic overlap.7 This promotes the efficient differentiation of risk categories, creating more defined boundaries for the classification of OPMDs at greatest malignant risk. Algorithmic calculations enable Straticyte™ to determine a specific risk range for each graded category, with a high score signifying a progression probability of greater than 55% over five years.7
Experimental use of Straticyte™ has illustrated a promising capability to accurately reclassify OPMDs of mild and moderate dysplasia (and uncertain risk) into defined low- or high-risk categories.7 This increase in the precise understanding of a patient’s oral cancer risk could ultimately guide clinicians to a more confident and supported treatment plan, favouring early detection and preventive action. It should be emphasized that additional exploratory studies are required to validate standard incorporation of Straticyte™ into the clinical assessment of cancer risk in oral lesions.
Conclusion
Future implementation of Straticyte™ (or other biomarker tests) into the clinical risk assessment of suspicious oral lesions should be used to complement, not replace, current methods of traditional dysplasia grading. Clinician appraisal of OPMD cancer risk can be strengthened by routine consideration of three fundamental assessment parameters: i) patient history, ii) clinical examination and iii) surgical biopsy, including histopathologic and biomarker analysis3,7 (Fig. 4).
Fig. 4
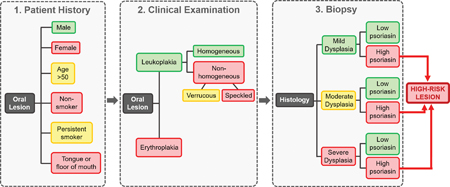
The combined evaluation of multiple preneoplastic components can provide a more holistic, objective and assured assessment of OSCC risk, to better guide treatment. In the scenario where a patient presents with an OPMD of mild dysplasia but excessive amounts of psoriasin: an increased certainty of lesion progression is established, motivating the clinician to attentively monitor, or definitively remove, the dysplastic region.7 A case of severe dysplasia with high psoriasin would signify extreme malignant risk, prompting immediate excision of the lesion. OPMDs of severe dysplasia and low psoriasin may warrant continued surveillance.7 Incorporation of a prognostic biomarker(s), like psoriasin, into the diagnostic toolkit of OPMDs is on the imminent horizon, with great potential to heighten early cancer detection and improve patient outcomes.
Oral Health welcomes this original article.
References
- El-Naggar AK, Chan JKC, Rubin Grandis J, Takata T, Slootweg PJ, International Agency for Research on Cancer. WHO Classification of Head and Neck Tumours.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016; 66(1):7–30.
- Speight PM, Khurram SA, Kujan O. Oral potentially malignant disorders: risk of progression to malignancy. Oral Surg Oral Med Oral Pathol Oral Radiol. 2018; 125(6):612–627.
- Warnakulasuriya S, Johnson NW, Van Der Waal I. Nomenclature and classification of potentially malignant disorders of the oral mucosa. J Oral Pathol Med. 2007; 36(10):575–580.
- Warnakulasuriya S, Ariyawardana A. Malignant transformation of oral leukoplakia: A systematic review of observational studies. J Oral Pathol Med. 2016; 45(3):155–166.
- Darling MR, Hassan A, Mclean L. Psoriasin: A new biomarker in the identification of cancer risk in oral lesions. Oral Health. December 2018.
- Hwang JTK, Gu YR, Shen M, et al. Individualized five-year risk assessment for oral premalignant lesion progression to cancer. Oral Surg Oral Med Oral Pathol Oral Radiol. 2017; 123(3):374–381.
- Warnakulasuriya S, Reibel J, Bouquot J, et al. Oral epithelial dysplasia classification systems: Predictive value, utility, weaknesses and scope for improvement. J Oral Pathol Med. 2008; 37(3):127–133.
- Abbey LM, Kaugars GE, Gunsolley JC, et al. Intraexaminer and interexaminer reliability in the diagnosis of oral epithelial dysplasia. Oral Surg Oral Med Oral Pathol Oral Radiol. 1995; 80(2):188–191.
- Pindborg JJ, Reibel J, Holmstrup P. Subjectivity in evaluating oral epithelial dysplasia, carcinoma in situ and initial carcinoma. J Oral Pathol Med. 1985; 14(9): 698–708.
- Warnakulasuriya S, Kovacevic T, Madden P, et al. Factors predicting malignant transformation in oral potentially malignant disorders among patients accrued over a 10-year period in South East England. J Oral Pathol Med. 2011; 40(9):677–683.
- Yan F, Reddy PD, Nguyen SA, et al. Grading systems of oral cavity pre-malignancy: a systematic review and meta-analysis. Eur Arch Otorhinolaryngol. May 2020 [published online ahead of print].
- Kujan O, Oliver RJ, Khattab A, et al. Evaluation of a new binary system of grading oral epithelial dysplasia for prediction of malignant transformation. Oral Oncol. 2006; 42(10):987–993.
- Smith J, Rattay T, McConkey C, et al. Biomarkers in dysplasia of the oral cavity: A systematic review. Oral Oncol. 2009; 45(8):647–653.
- Tripathi SC, Matta A, Kaur J, et al. Nuclear S100A7 is associated with poor prognosis in head and neck cancer. PLoS One. 2010; 5(8):e11939.
- Broome AM, Ryan D, Eckert RL. S100 protein subcellular localization during epidermal differentiation and psoriasis. J Histochem Cytochem 2003; 51(5):675–685.
- Wolf R, Ruzicka T, Yuspa SH. Novel S100A7 (psoriasin)/S100A15 (koebnerisin) subfamily: Highly homologous but distinct in regulation and function. Amino Acids. 2011; 41(4):789–796.
- Kesting MR, Sudhoff H, Hasler RJ, et al. Psoriasin (S100A7) up-regulation in oral squamous cell carcinoma and its relation to clinicopathologic features. Oral Oncol. 2009; 45(8):731–736.
- Jia J, Duan Q, Guo J, et al. Psoriasin, a multifunctional player in different diseases. Curr Protein Pept Sci. 2014; 15(8):836–842.
About the Author
 Victoria Thorburn is currently a second-year dentistry student at the Schulich School of Medicine and Dentistry at Western University, London, Ontario; mentored this past summer by Dr. Dong and Dr. Darling as a participant of the Schulich Dentistry Research Opportunity Program. Dr. Cecilia Dong is an Assistant Professor in the Division of Prosthodontics, Schulich School of Medicine and Dentistry at Western University, where she is also the Dentistry Lead for Interprofessional Education. She is cross-appointed to the Department of Pathology and Laboratory Medicine and the Department of Otolaryngology – Head and Neck Surgery. Dr. Mark Darling is Professor, Division of Oral Pathology in the Department of Pathology and Laboratory Medicine, Schulich School of Medicine and Dentistry at Western University. He currently serves on the editorial boards of the journals Oral Surgery Oral Medicine Oral Pathology Oral Radiology, Head and Neck Pathology and Case Reports in Dentistry. He is also an Associate Editor for the Journal of Investigative and Clinical Dentistry.
Victoria Thorburn is currently a second-year dentistry student at the Schulich School of Medicine and Dentistry at Western University, London, Ontario; mentored this past summer by Dr. Dong and Dr. Darling as a participant of the Schulich Dentistry Research Opportunity Program. Dr. Cecilia Dong is an Assistant Professor in the Division of Prosthodontics, Schulich School of Medicine and Dentistry at Western University, where she is also the Dentistry Lead for Interprofessional Education. She is cross-appointed to the Department of Pathology and Laboratory Medicine and the Department of Otolaryngology – Head and Neck Surgery. Dr. Mark Darling is Professor, Division of Oral Pathology in the Department of Pathology and Laboratory Medicine, Schulich School of Medicine and Dentistry at Western University. He currently serves on the editorial boards of the journals Oral Surgery Oral Medicine Oral Pathology Oral Radiology, Head and Neck Pathology and Case Reports in Dentistry. He is also an Associate Editor for the Journal of Investigative and Clinical Dentistry.












